Abstract
The distinction between Sézary syndrome (SS) and benign erythrodermic inflammatory diseases (EID) is difficult to make both clinically and on skin biopsies, since histomorphology can provide non specific results. New markers of circulating malignant Sézary cells have been recently described, especially CD158k/KIR3DL2 and T-plastin, but it has not been yet determined whether they could help for the diagnosis of erythroderma in skin samples. In this study, 13 frozen skin specimens from 10 SS patients and 26 from EID were analyzed for CD158k/KIR3DL2 expression using immunohistochemistry with AZ158 monoclonal antibody (moAb), which also recognizes the monomeric CD158e/KIR3DL1 receptor. Although positive in all SS samples, immunohistochemistry appeared not to be enough discriminant between SS and EID. Therefore in all samples disclosing a significant staining with AZ158 moAb, CD158k/KIR3DL2, CD158e/KIR3DL1 and T-plastin mRNA expression were analyzed on the same skin specimen using conventional and/or quantitative real time RT-PCR. Interestingly, only CD158k/KIR3DL2 transcripts were found to be significantly overexpressed in skin biopsies from patients with SS (P<.0001), including when normalization to CD3 expression was achieved (P=.0003). In light of these findings, CD158k/KIR3DL2 transcripts appear to be a unique molecular marker of SS in skin samples, allowing differential diagnosis with benign EID in routine practice.
Keywords: Aged, Alternative Splicing, Antibodies, Monoclonal, diagnostic use, Biopsy, Cryopreservation, Dermatitis, Exfoliative, diagnosis, pathology, physiopathology, Diagnosis, Differential, Female, Humans, Immunohistochemistry, Male, Middle Aged, Phosphoproteins, genetics, RNA, Messenger, metabolism, Receptors, KIR2DL2, genetics, metabolism, Sezary Syndrome, diagnosis, pathology, physiopathology, Skin, pathology, Skin Neoplasms, diagnosis, pathology, physiopathology, Tumor Markers, Biological, genetics
Keywords: Sézary syndrome, erythroderma, CD158k/KIR3DL2, T-plastin, RT-PCR
Introduction
Erythroderma is a severe dermatological condition, which may result from different causes, including cutaneous T-cell lymphoma (CTCL), especially Sézary syndrome (SS), and several erythrodermic inflammatory diseases (EID), mostly psoriasis, drug-eruptions or atopic dermatitis (Akhyani et al, 2005; Pal and Haroon, 1998; Rym et al, 2005). In the clinical setting of erythroderma, the identification of an underlying cause is difficult in practice, since clinical and histopathological aspects are most often not enough specific. It is necessary to differentiate benign EID from SS, which is an agressive lymphoma that requires an early and appropriate management (Willemze et al., 2005). SS is characterized by the presence of a malignant CD4+CD45RO+ T-cell clone that localizes in both the blood and skin. In SS, cutaneous and circulating malignant cells can be roughly identified by morphological analyzes. Circulating Sezary cells however are not specific for the diagnosis of SS (Vonderheid et al., 1985) and histopathologically, atypical epidermotropic T-cells, when present, are sometimes difficult to identify in skin biopsies. The identification of a monoclonal T-cell population can provide diagnostic informations (Curco et al., 1997; Gorochov et al., 1995; Guitart and Kaul, 1999; Witzens et al., 1997; Wood et al., 1994), which, however, are not fully specific (Delfau-Larue et al., 2000). Besides the lack of CD26 expression (Bernengo et al., 2001; Jones et al., 2001; Scala et al., 2002) and CD7 (Rappl et al., 2001), we have described new cell surface markers of malignant Sézary cells (Bagot et al., 2001; Huet et al., 2006). Among them, CD158k/KIR3DL2, a killer immunoglobulin-like receptor normally expressed by minor subsets of circulating T CD8+ lymphocytes and natural killer (NK) cells (Moretta et al., 1997), readily allows the identification of the malignant T-cells in the blood of SS patients (Ortonne et al., 2006; Poszepczynska-Guigne et al., 2004).
Recent studies based on genomic and cDNA microarrays technologies have identified new molecular markers of Sézary cells, such as JUNB gene amplification and overexpression (Mao et al.. 2004: Mao et al., 2003), NAV3 gene deletion (Karenko et al.. 2005) and abnormal expression of various markers at the transcript and/or protein level, such as Twist, and EphA4 (Kari et al., 2003: Nebozhyn et al., 2006). Among them, T-plastin is a member of the Fimbrins/plastins family, which are highly conserved actin-bundling proteins. T-plastin is not expressed by normal lymphocytes under physiological conditions and was therefore proposed as a specific molecular marker for neoplastic lymphocytes in SS, but, to the best of our knowledge, was not investigated in lesional skin (Su et al., 2003).
Importantly, only few studies to date have focused on cutaneous samples, so that a specific marker for SS in skin biopsies is still lacking. Although KIR3DL2/CD158k actually delineates the malignant clonal T-cell population in SS (Bagot et al., 2001; Ortonne et al., 2006; Poszepczynska-Guigne et al., 2004) and is expressed at the protein level by lymphocytes in erythrodermic skin of patients with SS (Wechsler et al., 2003), it is not known whether it may help for the diagnosis of erythroderma in skin biopsies. To address this issue, we analyzed the expression of CD158k/KIR3DL2 at both protein and transcript levels in a panel of skin biopsies from patients with erythroderma from various causes, and we compared the results obtained in SS versus benign EID.
Results
Immunohistochemistry with AZ158 moAb did not clearly discriminate between SS and benign EID
We observed a subepidermal band-like or mostly perivascular lymphocytic infiltrate in all 40 skin samples from the SS and the EID groups, associated with epidermotropic lymphocytes in 85%. The density of dermal T-cell infiltrates was categorized as +, ++ and +++ in 0 (0%), 8 (57%) and 6 (43%) samples from the SS group, respectively, and in 11 (42%), 12 (46%) and 3 (12%) samples from the EID group, respectively. This indicated a higher lymphocytic infiltration in SS, as shown in the cutaneous sample of patient 8 in Figure 1c. As previously reported (Wechsler et al., 2003), immunohistochemistry with AZ158 moAb was positive in all skin biopsies from patients with SS (Figure 1e), in agreement with CD158k/KIR3DL2 expression. However, with AZ158 moAb, we also found positive cells in 17 biopsies of the EID group (65%), as shown in figure 1f, showing representative sections of a patient with erythrodermic psoriasis. Mean AZ158/CD3 ratios were similar in both groups in the epidermis. However, in EID we found a lower staining with AZ158 moAb in the dermis, with 33% of SS samples versus 74% of EID samples showing a AZ158/CD3 ratio <25%, (Student’s t test: P = 0.0045). SS displayed lower CD8/CD3 ratios in both the epidermis and the dermis. Indeed, the mean CD8/CD3 ratio was 12% in SS versus 38 % in EID (Student’s t test: P = 0.003) in the epidermis. In the dermis, semi-quantitative evaluation revealed that 83% of SS versus 38% of EID samples had a CD8/CD3 ratio <25% (Student’s t test: P = 0.004). This latter finding is in agreement with the preferential intra-epidermal localization of malignant CD4+ lymphocytes in epidermotropic CTCL such as SS and mycosis fungoides, whereas many reactional CD8+ lymphocytes are present in the dermis (Bagot et al., 1992; Ortonne et al., 2003). In addition, in all investigated cases, we did not find CD56+ lymphocytes (data not shown), indicating that NK cells were not predominant within the infiltrates. Altogether, these results indicated that a higher T-cell infiltration was present in SS than in EID skin samples, and that differential diagnosis between SS and EID can not be done reliably using immunohistochemistry with AZ158moAb on frozen skin sections.
Figure 1. Immunohistochemistry results for CD3 and CD158k/e (AZ158 moAb) in representative SS and EID skin samples.
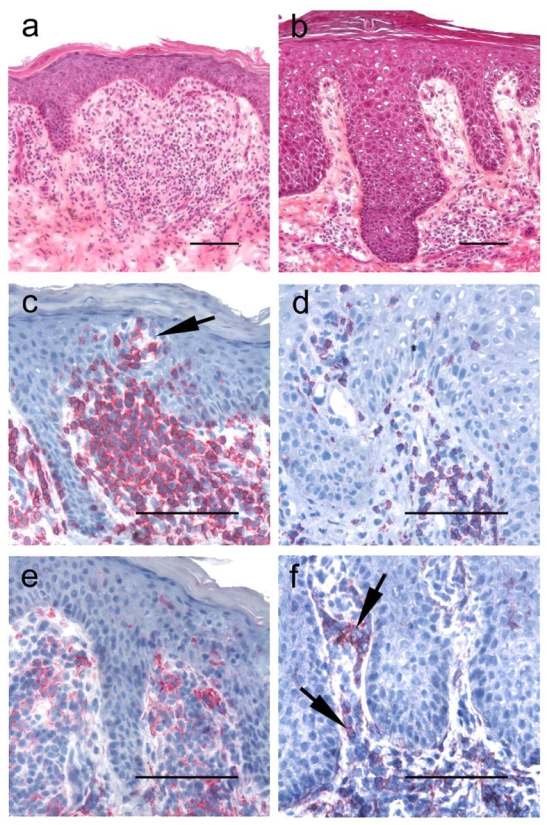
Histology and CD3 and AZ158 moAb stainings in cutaneous biopsies from the representative SS patient 8 (panels a, c, e), and from a patient with erythrodermic psoriasis (panels b, d, f) Hematoxylin and eosin-stained sections show in both samples a sub-epidermal band-like lymphocytic infiltrate associated with scattered epidermotropic lymphocytes. CD3+ T-cells (c–d), but a higher T-cell infiltrate is present in the SS sample (c), associated with Pautrier microabcesses (arrow). Lymphocytes stained with AZ158 moAb are seen in both the dermis and the epidermis in the SS sample (e), but also in a significant proportion of dermal lymphocytes (arrows) in the erythrodermic psoriasis cutaneous sample (f). Slides are shown at ×200 original magnification in panels a and b and ×400 for panels c–f. Bar = 0.1mm.
CD158k/KIR3DL2 transcripts were significantly overexpressed in SS versus benign EID skin samples
Using conventional RT-PCR CD158k/KIR3DL2 transcripts were readily detected in all but one biopsies from the SS group (89%) whereas significant expression of CD158k/KIR3DL2 mRNA could not be evidenced in any sample from the EID group. Representative results from conventional RT-PCR are shown in Figure 2. Using this technique, a distinct bright band could be visualized on agarose gel electrophoresis in 8/9 biopsies from the SS group (Figure 2b middle and right panels), with a similar expression to normal NK cells and OGU Sézary cell line (Figure 2a, left and middle panels). By contrast, a negative result or non specific bands were obtained in all samples from the EID groups (Figure 2b, left panel). CD158e/KIR3DL1 mRNA were never detected in both the SS and EID groups (Figure 2b).
Figure 2. Conventional RT-PCR amplification of CD158e/KIR3DL1 and CD158k/KIR3DL2 in erythrodermic skin samples.
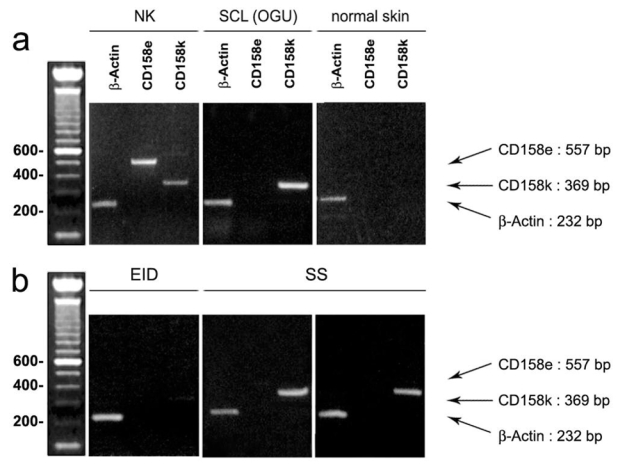
(a) Controls. In natural killer lymphocytes from a healthy donor, used as a positive control, both CD158e/KIR3DL1 and CD158k/KIR3DL2 transcripts are found (left), whereas in the Sézary cell line OGU, only CD158k/KIR3DL2 appears to be positive (middle). By contrast, in normal skin used as a negative control, both CD158e and CD 158k RT-PCR are negative (right), (b) Skin biopsies from patients with erythroderma.. In the skin sample from the EID group, neither CD158e/KIR3DL1 nor CD158k/KIR3DL2 mRNA expression is detected (left). By contrast, in the two SS samples (middle and right), a significant expression of CD158k/KIR3DL2 is found, with a bright distinctive band at the expected size, whereas CD158e/KIR3DL1 transcript are not detected.
Using quantitative RT-PCR, CD3 mRNA expression was detected in both SS and EID skin samples, indicating the presence of a significant subset of T lymphocytes within the cutaneous infiltrate in both groups (Fig. 3a, left panel). We found a slight CD3 transcript over-expression in SS samples (Mann-Whitney’s test: P=0.0133), in agreement with results of morphological analyzes indicating that T-cell infiltration was higher in the SS group. Using quantitative RT-PCR, we found, with a different set of primers specifically designed for the study (Table S2, lower part), that CD158k/KIR3DL2 mRNA were readily detected in all but one SS skin samples, with a significant over-expression when compared to samples from the EID group (Mann-Whitney’s test: P<0.0001), as shown in figure 3a, middle panel. In EID skin samples, CD158k/KIR3DL2 mRNA appeared to be either undetectable (n = 6, 60%) or expressed at very low levels (n = 4, 40%) as for one sample from the SS group (patient 7, Fig. 3b, left panel) so that quantification was considered inappropriate, due to the presence of non specific products on control agarose gel electrophoresis. CD158e/KIR3DL1 transcripts were undetectable in both the SS and the EID groups (data not shown). To avoid a possible misinterpretation related to the higher T-cell infiltration found in SS skin samples, we also evaluated the CD158k/CD3 ratio of relative mRNA copy number, which remained significantly higher in the SS versus the EID group (Mann-Whithney’s test: P=0.0003).
Figure 3. Quantitative RT-PCR amplification of CD3, CD158k/KIR3DL2 and T-plastin in erythrodermic skin samples.
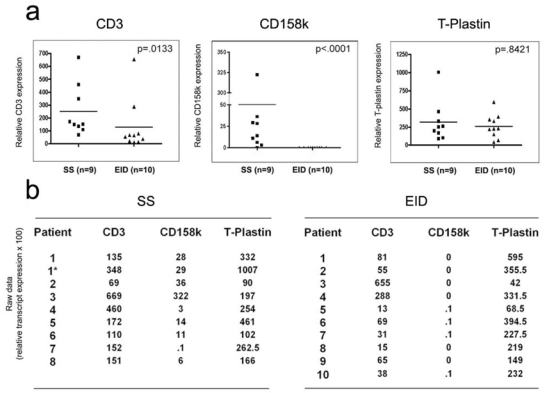
Activated PBMCs from a healthy control donor were used as a calibrator and arbitrarily set as a CD3, CD158e/KIR3DL1, CD158k/KIR3DL2 and T-plastin expression level of 1. The middle bar in each group represents the median of that group, (a) The median level of CD3 is higher in the SS group (P=0.0133) (left). CD158k/KIR3DL2 mRNA expression is highly overexpressed in SS versus EID skin samples (P<0.0001) (middle). By contrast, T-plastin transcript quantification is not discriminant between SS and EID (P=0.8421) (right), (b) Raw data (relative transcript expression ×100), showing a significant overexpression of CD158k/KIR3DL2 mRNA but not T-plastin in SS (left) versus EID (right) skin samples. All data points represent the average of duplicate tests.
Interestingly, in patient 1, we could detect CD 158k transcripts in a skin sample taken at a pre-erythrodermic stage of the disease. This patient had erythematous and squamous plaques with only few circulating CD158k/KIR3DL2+CD4+ T-cells and further developped erythroderma. Patients 6 and 4 were evaluated at the stage of partial remission under treatment (Table S1). Patient 6 partially responded to interferon α with persistent erythemato-squamous plaques. In this patient, CD158k/KIR3DL2 expression could be demonstrated using AZ158 immunostaining and RT-PCR studies, indicating that malignant T-cells were present in the skin, whereas only few Sézary cells could be detected in the blood. Patient 4 had TNM stage IV disseminated disease and was evaluated after he was placed under systemic polychemotherapy (patient 4, Table S1). At this stage, no circulating CD4+CD158k/KIR3DL2+ cells were found in the blood, in agreement with successful lysis of tumoral cells by efficient chemotherapy, but he had a slight persistent erythroderma. Interestingly, AZ158 stained cells together with a positive RT-PCR for CD158k/KIR3DL2 were found in the skin from this patient. These results suggested that although malignant T-cells could not be detected in the blood, viable tumoral cells were present in the skin.
Altogether, these findings show that the detection of CD158k/KIR3DL2 transcripts in the skin allows to distinguish SS from benign erythrodermic inflammatory dermatoses, thus indicating that CD158k/KIR3DL2 is a reliable molecular marker of SS in skin samples. Furthermore, results obtained in patients 1, 4 and 6 suggest that CD158k/KIR3DL2 mRNA detection in the skin may allow diagnosis of SS even at pre-erythrodermic early stage and may help for the detection of residual disease under treatment.
T-plastin transcripts expression was not significantly higher in SS versus benign EID skin samples
T-plastin, a member of the fimbrin/plastin family normally expressed at low levels by nonhaematopoietic mesenchymal and epithelial tissues (Lin et al., 1993), has been recently shown to delineate circulating malignant Sézary cells (Su et al., 2003). Although T-plastin is expected to be expressed in normal skin, we sought to verify whether it might be overexpressed in erythrodermic skin of SS patients, due to infiltration of T-plastin expressing neoplastic lymphocytes. Using quantitative RT-PCR, we found that T-plastin transcripts were detected at similar levels in cutaneous samples from the SS and the EID group, as shown in Figure 3a, right panel (Mann-Whitney’s test: P = 0.8421). The T-plastin/CD3 relative mRNA copy number ratio appeared to be slightly more elevated in the EID group, but the difference was not statistically significant (Mann-Whitney’s test: P = 0.02). These results suggest that, contrasting with CD158k, T-plastin is not a reliable molecular marker for the diagnosis of SS in skin biopsies.
CD158k/KIR3DL2 alternatively spliced variants were not detected in skin samples of benign EID
In the present study, we obtained in some skin samples conflicting results with immunohistochemistry and RT-PCR regarding CD158k/KIR3DL2 expression. Indeed, immunoreactivity with AZ158 moAb was found within lymphocytic infiltrates of a proportion of EID, while we could not detect significant expression of CD158k/KIR3DL2 transcript in the same skin specimen. One hypothesis is that non neoplastic reactional lymphocytes in erythrodermic skin from patients with benign dermatoses may express alternative splicing variant of CD158k/KIR3DL2, lacking one or both of the exonic domains of primer hybridization. To address this issue, we designed three additional sets of primers, allowing amplification of three distinct CD158k/KIR3DL2 transcript segment located within exon 3 – 5,4–9, and 8–9 (Table S2, Figure 4). Using all three sets of primers, negative results were constantly obtained in all four EID samples analyzed, as well as in normal skin used as a negative control. By contrast, we could detect a distinct band at the expected size on agarose gel electrophoresis in both skin samples from patient 3 and Sezary cell line OGU, used as a positive control. These findings indicate that the negative results obtained with both conventional and quantitative RT-PCR regarding CD158k/KIR3DL2 mRNA expression in the EID group were not due to alternative splicing of CD158k/KIR3DL2 transcripts.
Figure 4. KIR3DL2 gene, RT-PCR products and primers localization.

Relative positions of the different cDNA segments (bottom) across the 9 exons of the KIR3DL2 gene (top), amplified acording to the different sets of primers used for conventional and quantitative RT PCR.
Discussion
Histopathological diagnosis of SS is often difficult, unless distinct atypical epidermotropic T-cells are identified. It is hampered by its morphologic similarity to inflammatory dermatoses and the low proportion of tumoral cells, which often account for a minority of the total tissue cells. In addition, with the exception of Twist and EphA4 transcripts (van Doom et al., 2004), none of the newly described molecular marker of malignant Sézary cells has been evaluated in skin samples. We have previously demonstrated that CD158k/KIR3DL2 allows the identification of malignant Sézary cells in the blood, but the diagnostic value of this marker for the diagnosis of erythroderma in skin specimen has never been studied. In the present study, expression of CD158k/KIR3DL2 was analyzed at both protein product and transcriptional levels in a series of frozen skin biopsies from patients with erythroderma. Since AZ158 moAb, which recognizes both CD158k/KIR3DL2 and CD158e/KIR3DL1, was used for immunohistochemical procedures, both transcripts were analyzed by molecular studies on the same skin specimen. Unexpectedly, we found that, although constantly positive in SS samples, immunohistochemistry using AZ158 moAb in frozen skin sections was not enough discriminant between SS and EID. Immunoreactivity to AZ158 moAb was actually demonstrated in a significant proportion of benign inflammatory erythrodermas, suggesting that CD158k/KIR3DL2+ and/or CD158e/KIR3DL1+ cells are recruited in the skin in erythrodemic inflammatory conditions. As NK lymphocytes and minor subsets of CD8+ T-cells may express KIRs under physiological conditions (Moretta et al., 1997), it is tempting to hypothesize that positive cells in the EID group corresponded to normal lymphocytes expressing CD158k/KIR3DL2 and/or CD158e/KIR3DL1. The higher CD8/CD3 ratios and the constant negativity of CD56 staining obtained in the EID group supports the hypothesis that these cells might be indeed reactive CD8+ T lymphocytes. Surprisingly, using both conventional and quantitative RT-PCR experiments, we found a significant overexpression of CD158k/KIR3DL2 transcripts in skin samples of the SS versus the EID group. By contrast, quantitative RT-PCR experiments revealed that the level of T-plastin expression was similar in both groups. This result probably reflects that other, non lymphoid, cells express T-plastin in the skin, in agreement with its known synthesis by a wide variety of non haematopoietic epithelial and non epithelial cells (Lin et al., 1993). As we found evidences that SS had a higher T-cell infiltration than EID, CD158k/KIR3DL2 and T-plastin relative mRNA expression was normalized to CD3, which still showed a significant overexpression of the former in the SS group. In this series, using specific transcripts detection and quantification, CD158k/KIR3DL2 thus appeared to be a unique molecular marker of SS in the skin, contrasting with immunohistochemistry with AZ158 moAb and T-plastin mRNA quantification, which were not enough discriminant between SS and benign EID. Noted that, two patients from this series were evaluated at the stage of partial remission under treatment. One partially responded to interferon α with persistent erythemato-squamous plaques whereas the other one had advanced disease and was evaluated after he was placed under systemic PCT. In skin biopsies of these patients, CD158k/KIR3DL2 expression could be demonstrated using AZ158 immunostaining and RT-PCR assays, whereas no or few Sézary cells could be detected in the blood. This finding suggested that although malignant T-cells could not be detected in the blood, viable tumor cells were still present in the skin. In light of these results, it is tempting to speculate that the skin may represent a sanctuary site for neoplastic cells in patients placed under systemic therapies. In agreement with this hypothesis, Assaf et al. have recently reported a patient with erythrodermic SS who turned into plaque stage disease and then high grade T-cell lymphoma under treatment with extracorporeal photopheresis, whereas clearance of circulating Sézary cells was obtained (Assaf et al., 2004). They however found an identical dominant T-cell clone in the skin at all stages, suggesting that malignant cells were still present in the skin under treatment and further transformed in situ. In this context, we can ask whether CD158k/KIR3DL2 specific RT-PCR performed on skin biopsies could be a unique tool for the evaluation of residual disease under treatment.
Interestingly, in one patient with SS, we could detect CD158k/KIR3DL2 transcripts in a skin sample taken at a pre-erythrodermic stage of the disease. Initially, this patient presented with erythematous and squamous plaques with only few circulating CD158k/KIR3DL2+CD4+ T-cells so that a diagnosis of mycosis fungoides was proposed. She further developed erythroderma with atypical CD158k/KIR3DL2+ circulating Sézary cells, allowing diagnosis of SS. This result suggests that CD158k/KIR3DL2 mRNA might be used as an early marker for SS, allowing identification of the malignant cells in the skin before development of erythroderma. Further studies are however needed to determine whether CD158k/KIR3DL2 mRNA expression may be used to differentiate early plaque stage SS from mycosis fungoides, characterized by an indolent course.
It is important to note that conflicting results were obtained with immunohistochemistry and RT-PCR in some cases from the BID group regarding CD158k/KIR3DL2 expression. AZ158 moAb indeed labelled the lymphocytic infiltrates in a proportion of cases whereas CD158k/KIR3DL2 transcripts appeared to be either undetectable or expressed at so low level that reliable quantification was not possible. CD158e/KIR3DLl transcripts were not found in any investigated case, thus ruling out the hypothesis that AZ158 labelled cells could be mostly CD158e/KIRDLl+ lymphocytes. We also conducted additional molecular studies in representative cases, which excluded the possibility that alternative splicing variants of CD158k/KIR3DL2, with skipping of an exonic domain of primer hybridization, were expressed in erythrodermic inflammatory conditions. Further studies are needed to determine whether infiltrating lymphocytes in benign erythrodermic dermatoses may express very low levels of KIRs transcripts, as compared to malignant Sézary cells, or may transiently activate the transcription of KIRs before recruitment in the skin. In agreement with this latter hypothesis, the heterogeneous expression of some KIRs within an identical T-cell clone has been previously reported in normal T-cells (Vely et al., 2001), and, similarly, we have reported heterogeneous expression of CD158k/KIR3DL2 within a malignant Sézary T-cell clone (Ortonne et al., 2006). These findings indeed indicate that KIRs expression in T-cells can occur at a mature stage of differentiation, after TCR rearrangements, and further suggest that KIR expression may be positively and/or negatively regulated in these cells.
In conclusion, the detection of significant levels of CD158k/KIR3DL2 transcript using quantitative RT-PCR in erythrodermic skin allows diagnosis of SS, even in very early stages of the disease, and represents a reliable marker of the disease in skin samples. This molecular marker can be easily used in routine practice to make differential diagnosis between erythrodermic benign inflammatory dermatoses and SS, which requires early and specific management.
Materials and methods
Patients selection, control cases and cells
Thirteen frozen biopsy samples from 10 patients with SS were retrieved from the files of the Department of Pathology of our institution (Hôpital Henri Mondor, Créteil). The clinical features of the patients with SS are shown in Table 1. There were 4 females and 6 males, with a median age of 71 years (range 61 – 79 years). In these patients, diagnosis of SS was made according to the current WHO-EORTC classification(Willemze et al., 2005) and following the criteria defined by Russell-Jones et al (Russell-Jones, 2005). Among these criteria, a T-cell clone could be evidenced in both the skin and blood of all patients from the SS group. In addition, CD158k/KIR3DL2 expression by peripheral blood lymphocytes could be demonstrated in 3 patients out of the SS group (patients 1, 2 and 3, Table 1) using flow cytometry studies, as previously described (Ortonne et al., 2006; Poszepczynska-Guigne et al., 2004). Two SS patients were investigated at the time of partial remission, with no or few circulating Sézary cells: patient 4 had slight persistent erythroderma without circulating Sézary cells after a first round of polychemotherapy (PCT) and patient 6 had persistant erythematous plaques under interferon α treatment. In the former, PCT was introduced because the patient had stage IV progressive disease with multiple large adenopathies.
As control samples, we selected skin biopsies from 26 erythrodermic patients (10 females and 16 males, mean age, 67 years), with a diagnosis of non-lymphomatous, benign EID: erythrodermic psoriasis (n=8), drug eruption (n=10 including one with acute generalized exanthematic pustulosis and three with drug-induced hypersensitivity syndrome), eczema (n=3), pityriasis rubra pilaris (n=1), myelodysplastic syndrome (n=1), primary hypereosinophilic syndrome (n=1) and unspecified erythroderma (n=2). T-cell clonality results were available in only 4 patients from the EID group. Among these 4 EID patients, a dominant T-cell clone was found in the skin in one and in the blood in another.
Normal skin samples and NK lymphocytes from healthy donors as well as the Sézary cell lines Pno, previously established in our laboratory (Poszepczynska et al., 2000), and OGU, recently developped (Marie-Cardine et al., 2007), were used as controls for molecular studies. NK lymphocytes were isolated from peripheral blood of a healthy donor using the magnetic-activated cell sorter (MACS) NK cell isolation kit (Miltenyi Biotec, Auburn, CA), following manufacturer’s intructions.
This study was approved by the Comité Consultatif de Protection des Personnes dans la Recherche Biomedicale institutional ethics committee. Informed consent was provided according to the Declaration of Helsinki.
Immunohistochemistry and evaluation of the CD8/CD3 and AZ158(CD158k/e)/CD3 ratios
For immunostaining procedure, 5 micrometer-thick frozen sections were applied on Superfrost plus slides (CML, Angers, France), overnight air dried and fixed in aceton for ten minutes before storing at −20°C. Slides were post-fixed in aceton for 5 minutes and air dried before use. The immunostaining procedure was performed after rehydratation in Tris/NaCl buffer. CD158k/KIR3DL2 expression was analyzed using the purified IgG2a AZ158 monoclonal antibody (moAb) (Innate Pharma, Marseille, France), which also recognizes the monomeric receptor CD158e/KIR3DL1(Parolini et al., 2002). The anti-CD3, -CD4, -CD8, and –CD56 moAbs were purchased from DAKO (DAKO SA, Glostrup, Denmark). All primary moAbs were used at a 1:50 dilution. We used an incubation period of 4 hours for AZ158 and 1 hour for all other primary moAbs, at room temperature. The staining was performed using a biotinylated secondary antibody and avidin conjugated to alkalin posphatase (vectastain ABC–AP kit from Vector, Burlingame, USA). The alkalin phosphatase reaction was revealed by Naphtol-Fast Red (Sigma-Aldrich, Saint Quentin Fallavier, France) and sections were counterstained in blue with hematoxylin. As a control for immunostaining with AZ158 moAb, we used frozen sections of normal spleen, where a subset of the scattered CD56+ NK cells normally present are expected to express CD158e/KIR3DL1 and/or CD158k/KIR3DL2.
In all samples, we categorized the density of dermal T-cell lymphocytic infiltrates into 3 groups: + scattered lymphocytes, ++ clusters of lymphocytes and +++ dense and diffuse infiltration, according to the CD3 staining. Epidermal and dermal lymphocytic infiltrates were separately analyzed. In the epidermis, the number of CD3+, CD4+, CD8+ and AZ158+ cells was counted on the total epidermal length. In the dermis, a semi-quantitative analysis was done, in which we categorized the CD4/CD3, CD8/CD3 and AZ158/CD3 ratios into 4 groups: 1 (0–25%), 2 (>25–50%), 3 (>50–75%) or 4 (>75%–100%).
RT-PCR amplification of CD3, CD158k/KIR3DL2, CD158e/KIR3DLl and T-plastin transcripts
Molecular studies were performed on the same frozen skin sample used for immunohistochemistry in 9 specimens from 8 patients out of the SS group and in 10 specimens out of the EID group. As controls for molecular studies, we used total mRNA extracted from normal skin and NK lymphocytes of healthy donors and from the Sézary cell lines Pno (Poszepczynska et al., 2000), previously established in our laboratory, and OGU, recently developped (Marie-Cardine et al., 2007).
For total RNA extraction from skin samples, twenty 50 micrometer-thick frozen sections were transferred into Trizol and immediately homogenized twice for 2 minutes with a Mixer Mill MM301 (Verder, Erahny sur Oise, France) before chloroform/isopropanol precipitation. Total mRNA was then reverse transcribed by using oligo-dT primers and Powerscript reverse transcriptase (RT Clontech, Palo Alto, CA).
Amplification of CD158k, CD158e and β-actin transcripts using conventional polymerase chain reaction (PCR) was performed as previously described (Uhrberg et al., 1997), with amplification conditions as following: initial denaturation at 95°C for 2 min; then 60 s at 62°C for hybridization, 45 s at 72°C for elongation and 60 s at 94°C for next steps denaturation, for the first five cycles, and then 45 s at 60°C, 45 s at 72°C and 30 s at 94°C for 30 cycles. We used previously reported primers for CD158k/KIR3DL2 and CD158e/KIR3DL1 (Uhrberg et al., 1997), as well as for β-actin (Ju et al., 1999), used as a positive control. Regarding CD158k/KIR3DL2, additionalsets of primers were used, in order to search for alternatively spliced transcripts, allowing amplification of mRNA segments located with exons 3–5, 4–9, and 8–9, as shown in Figure 1. The sequence and positions of all primers used for conventional PCR are indicated in Table S2 (upper part).
Quantitative PCR reactions for CD3 (delta chain), CD 158k, CD158e and T-plastin were performed in a LightCycler 2.0 System (Roche Diagnostics, Meylan, France) using a SYBR Green PCR kit from Roche Diagnostics (Meylan, France). Melting curves and agarose gel electrophoresis established the purity of the amplified product. Normalization was achieved by quantification of the mRNA expression of the SF3A1 gene, encoding for the 120 kDa subunit of the splicing factor 3a (Tanackovic and Kramer, 2005), chosen as control housekeeping gene (Szabo et al., 2004) for its stable expression in lymphocytes, as previously described (Mesel-Lemoine et al., 2006). PCR samples contained 4 mM MgCL2, 0.4μM of each primer, and amplification cycling conditions were as following: 94°C for denaturation, 10 seconds at 60°C for hybridization and 25 seconds at 72°C for elongation for 40 cycles. The expression of transcripts was measured by the relative quantification of real time-PCR, as previously described (Gibson et al., 1996). As calibrator samples, we used PBMC of healthy control donors activated for 1 hour with phorbol ester and ionomycin (5ng/ml and 1ng/ml, respectively) for CD3, CD 158k, CD158e, and a liver specimen for T-plastin. All PCR conditions were adjusted in order to obtain equivalent optimal amplification efficiency between the different assays. By using the obtained linear graphs, the differences in Ct values were determined for each sample and were expressed as relative percentage of mRNA present in the calibrator sample, according to the ΔΔCt method, after adjustment of PCR efficiency with the Light Cycler software 4.0 (Roche). Quantification was considered to be unreliable when the presence of non specific products was detected on the control agarose gel. In these cases, the relative percentage of transcripts was arbitrarily set as .1. All PCR experiment were done in duplicate. The sequence and positions of primers used for quantitative PCR are indicated in Table S2 (lower part).
T-cell clonality analysis
For T-cell clonality studies, DNA was extracted from a different snap frozen skin biopsy and analyzed by DNA amplification of the TCR-gamma gene using consensus primers and separation of the amplimers on denaturing sequencing gel (polymerase chain reaction-denaturing gradient gel electrophoresis), as previously described (Theodorou et al., 1995).
Statistical analysis
Student’s t test was used to compare the mean epidermal and dermal CD8/CD3 and AZ158/CD3 ratios between samples from the SS and the control EID groups. The non-parametric Mann-Whitney’s test was used because of the non-normal distributions of mRNA levels differences of CD3, CD158e/KIR3DL1, CD158k/KIR3DL2 and T-plastin expression between the SS and the EID specimens. Following Bonferroni correction for multiple testing with quantitative RT-PCR, differences were considered to be statistically significant at P<0.01.
Supplementary Material
Supplemental data is available at the Journal of Investigative Dermatology’s website
This table describes the clinical features of the patients with Sézary syndrome included in the study, including criteria used for the diagnosis of SS, such as the percent of Sézary cells, the percent of CD158k+ circulating lymphocytes and T-cell clonality results.
In this table are listed all the sequences of the primers used for both conventional and quantitative RT-PCR experiments : housekeeping genes (β-actin for conventional RT-PCR and SF3A1 for quantitative RT-PCR), CD3 (delta chain) CD158e, CD158k and T-plastin (PLS3 gene)
Acknowledgments
This work was supported by grants from the INSERM; Université Paris XII; Société Française de Dermatologie; Société de Recherche Dermatologique and the Association pour la Recherche sur le Cancer.
Footnotes
N.O and S.L.G. contributed equally as first authors
Conflict of interest
The authors state no conflict of interest
References
- Akhyani M, Ghodsi ZS, Toosi S, Dabbaghian H. Erythroderma: a clinical study of 97 cases. BMC Dermatol. 2005;5:5. doi: 10.1186/1471-5945-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf C, Hummel M, Zemlin M, Steinhoff M, Geilen CC, Stein H, et al. Transition of Sezary syndrome into mycosis fungoides after complete clinical and molecular remission under extracorporeal photophoresis. J Clin Pathol. 2004;57:1325–1328. doi: 10.1136/jcp.2004.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot M, Moretta A, Sivori S, Biassoni R, Cantoni C, Bottino C, et al. CD4(+) cutaneous T-cell lymphoma cells express the p140-killer cell immunoglobulin-like receptor. Blood. 2001;97:1388–1391. doi: 10.1182/blood.v97.5.1388. [DOI] [PubMed] [Google Scholar]
- Bagot M, Wechsler J, Lescs MC, Revuz J, Farcet JP, Gaulard P. Intraepidermal localization of the clone in cutaneous T-cell lymphoma. J Am Acad Dermatol. 1992;27:589–593. doi: 10.1016/0190-9622(92)70227-7. [DOI] [PubMed] [Google Scholar]
- Bernengo MG, Novelli M, Quaglino P, Lisa F, De Matteis A, Savoia P, et al. The relevance of the CD4+ CD26− subset in the identification of circulating Sezary cells. Br J Dermatol. 2001;144:125–135. doi: 10.1046/j.1365-2133.2001.04014.x. [DOI] [PubMed] [Google Scholar]
- Curco N, Servitje O, Llucia M, Bertran J, Limon A, Carmona M, et al. Genotypic analysis of cutaneous T-cell lymphoma: a comparative study of Southern blot analysis with polymerase chain reaction amplification of the T-cell receptor-gamma gene. Br J Dermatol. 1997;137:673–679. [PubMed] [Google Scholar]
- Delfau-Larue MH, Laroche L, Wechsler J, Lepage E, Lahet C, Asso-Bonnet M, et al. Diagnostic value of dominant T-cell clones in peripheral blood in 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. Blood. 2000;96:2987–2992. [PubMed] [Google Scholar]
- Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- Gorochov G, Bachelez H, Cayuela JM, Legac E, Laroche L, Dubertret L, et al. Expression of V beta gene segments by Sezary cells. J Invest Dermatol. 1995;105:56–61. doi: 10.1111/1523-1747.ep12312560. [DOI] [PubMed] [Google Scholar]
- Guitart J, Kaul K. A new polymerase chain reaction-based method for the detection of T-cell clonality in patients with possible cutaneous T-cell lymphoma. Arch Dermatol. 1999;135:158–162. doi: 10.1001/archderm.135.2.158. [DOI] [PubMed] [Google Scholar]
- Huet D, Bagot M, Loyaux D, Capdevielle J, Conraux L, Ferrara P, et al. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J Immunol. 2006;176:652–659. doi: 10.4049/jimmunol.176.1.652. [DOI] [PubMed] [Google Scholar]
- Jones D, Dang NH, Duvic M, Washington LT, Huh YO. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115:885–892. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV. [DOI] [PubMed] [Google Scholar]
- Ju J, Pedersen-Lane J, Maley F, Chu E. Regulation of p53 expression by thymidylate synthase. Proc Natl Acad Sci U S A. 1999;96:3769–3774. doi: 10.1073/pnas.96.7.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karenko L, Hahtola S, Paivinen S, Karhu R, Syrja S, Kahkonen M, et al. Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue. Cancer Res. 2005;65:8101–8110. doi: 10.1158/0008-5472.CAN-04-0366. [DOI] [PubMed] [Google Scholar]
- Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, et al. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. J Exp Med. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Park T, Chen ZP, Leavitt J. Human plastin genes. Comparative gene structure, chromosome location, and differential expression in normal and neoplastic cells. J Biol Chem. 1993;268:2781–2792. [PubMed] [Google Scholar]
- Mao X, Orchard G, Lillington DM, Child FJ, Vonderheid EC, Nowell PC, et al. BCL2 and JUNB abnormalities in primary cutaneous lymphomas. Br J Dermatol. 2004;151:546–556. doi: 10.1111/j.1365-2133.2004.06106.x. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ. Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101:1513–1519. doi: 10.1182/blood-2002-08-2434. [DOI] [PubMed] [Google Scholar]
- Marie-Cardine A, Huet D, Ortonne N, Remtoula N, Le Gouvello S, Bagot M, et al. Killer cell Ig-like receptors CD158a and CD158b display a co-activatory function, involving the c-jun NH2-terminal protein kinase signaling pathway, when both expressed on malignant CD4+ T cells from a Sezary patient. Blood. 2007 doi: 10.1182/blood-2007-02-071993. in press. [DOI] [PubMed] [Google Scholar]
- Mesel-Lemoine M, Cherai M, Le Gouvello S, Guillot M, Leclercq V, Klatzmann D, et al. Initial depletion of regulatory T cells: the missing solution to preserve the immune functions of T lymphocytes designed for cell therapy. Blood. 2006;107:381–388. doi: 10.1182/blood-2005-07-2658. [DOI] [PubMed] [Google Scholar]
- Moretta A, Biassoni R, Bottino C, Pende D, Vitale M, Poggi A, et al. Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev. 1997;155:105–117. doi: 10.1111/j.1600-065x.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Nebozhyn M, Loboda A, Kari L, Rook AH, Vonderheid EC, Lessin S, et al. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood. 2006;107:3189–3196. doi: 10.1182/blood-2005-07-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortonne N, Buyukbabani N, Delfau-Larue MH, Bagot M, Wechsler J. Value of the CD8-CD3 ratio for the diagnosis of mycosis fungoides. Mod Pathol. 2003;16:857–862. doi: 10.1097/01.MP.0000084112.81779.BB. [DOI] [PubMed] [Google Scholar]
- Ortonne N, Huet D, Gaudez C, Marie-Cardine A, Schiavon V, Bagot M, et al. Significance of circulating T-cell clones in Sezary syndrome. Blood. 2006;107:4030–4038. doi: 10.1182/blood-2005-10-4239. [DOI] [PubMed] [Google Scholar]
- Pal S, Haroon TS. Erythroderma: a clinico-etiologic study of 90 cases. Int J Dermatol. 1998;37:104–107. doi: 10.1046/j.1365-4362.1998.00228.x. [DOI] [PubMed] [Google Scholar]
- Parolini S, Cantoni C, Castriconi R. The AZ158 mAb specifically reacts with p70 and p140 inhibitory NK receptors for HLA-B and HLA-A alleles. In: Mason D, Andre P, Bensussan, et al., editors. Leukocyte Typing. VII. Oxford Univ Press; Oxford: 2002. pp. 415–417. [Google Scholar]
- Poszepczynska E, Bagot M, Echchakir H, Martinvalet D, Ramez M, Charue D, et al. Functional characterization of an IL-7-dependent CD4(+)CD8alphaalpha(+) Th3-type malignant cell line derived from a patient with a cutaneous T-cell lymphoma. Blood. 2000;96:1056–1063. [PubMed] [Google Scholar]
- Poszepczynska-Guigne E, Schiavon V, D’Incan M, Echchakir H, Musette P, Ortonne N, et al. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004;122:820–823. doi: 10.1111/j.0022-202X.2004.22326.x. [DOI] [PubMed] [Google Scholar]
- Rappl G, Muche JM, Abken H, Sterry W, Tilgen W, Ugurel S, et al. CD4(+)CD7(−) T cells compose the dominant T-cell clone in the peripheral blood of patients with Sezary syndrome. J Am Acad Dermatol. 2001;44:456–461. doi: 10.1067/mjd.2001.110900. [DOI] [PubMed] [Google Scholar]
- Russell-Jones R. Diagnosing erythrodermic cutaneous T-cell lymphoma. Br J Dermatol. 2005;153:1–5. doi: 10.1111/j.1365-2133.2005.06706.x. [DOI] [PubMed] [Google Scholar]
- Rym BM, Mourad M, Bechir Z, Dalenda E, Faika C, Iadh AM, et al. Erythroderma in adults: a report of 80 cases. Int J Dermatol. 2005;44:731–735. doi: 10.1111/j.1365-4632.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Scala E, Narducci MG, Amerio P, Baliva G, Simoni R, Giovannetti A, et al. T cell receptor-Vbeta analysis identifies a dominant CD60+ CD26− CD49d− T cell clone in the peripheral blood of Sezary syndrome patients. J Invest Dermatol. 2002;119:193–196. doi: 10.1046/j.1523-1747.2002.18194.x. [DOI] [PubMed] [Google Scholar]
- Su MW, Dorocicz I, Dragowska WH, Ho V, Li G, Voss N, et al. Aberrant expression of T-plastin in Sezary cells. Cancer Res. 2003;63:7122–7127. [PubMed] [Google Scholar]
- Szabo A, Perou CM, Karaca M, Perreard L, Quackenbush JF, Bernard PS. Statistical modeling for selecting housekeeper genes. Genome Biol. 2004;5:R59. doi: 10.1186/gb-2004-5-8-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanackovic G, Kramer A. Human splicing factor SF3a, but not SF1, is essential for pre-mRNA splicing in vivo. Mol Biol Cell. 2005;16:1366–1377. doi: 10.1091/mbc.E04-11-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou I, Delfau-Larue MH, Bigorgne C, Lahet C, Cochet G, Bagot M, et al. Cutaneous T-cell infiltrates: analysis of T-cell receptor gamma gene rearrangement by polymerase chain reaction and denaturing gradient gel electrophoresis. Blood. 1995;86:305–310. [PubMed] [Google Scholar]
- Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- van Doom R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, et al. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- Vely F, Peyrat M, Couedel C, Morcet J, Halary F, Davodeau F, et al. Regulation of inhibitory and activating killer-cell Ig-like receptor expression occurs in T cells after termination of TCR rearrangements. J Immunol. 2001;166:2487–2494. doi: 10.4049/jimmunol.166.4.2487. [DOI] [PubMed] [Google Scholar]
- Vonderheid EC, Sobel EL, Nowell PC, Finan JB, Helfrich MK, Whipple DS. Diagnostic and prognostic significance of Sezary cells in peripheral blood smears from patients with cutaneous T cell lymphoma. Blood. 1985;66:358–366. [PubMed] [Google Scholar]
- Wechsler J, Bagot M, Nikolova M, Parolini S, Martin-Garcia N, Boumsell L, et al. Killer cell immunoglobulin-like receptor expression delineates in situ Sezary syndrome lymphocytes. J Pathol. 2003;199:77–83. doi: 10.1002/path.1251. [DOI] [PubMed] [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- Witzens M, Mohler T, Willhauck M, Scheibenbogen C, Lee KH, Keilholz U. Detection of clonally rearranged T-cell-receptor gamma chain genes from T-cell malignancies and acute inflammatory rheumatic disease using PCR amplification, PAGE, and automated analysis. Ann Hematol. 1997;74:123–130. doi: 10.1007/s002770050269. [DOI] [PubMed] [Google Scholar]
- Wood GS, Tung RM, Haeffner AC, Crooks CF, Liao S, Orozco R, et al. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE) J Invest Dermatol. 1994;103:34–41. doi: 10.1111/1523-1747.ep12389114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table describes the clinical features of the patients with Sézary syndrome included in the study, including criteria used for the diagnosis of SS, such as the percent of Sézary cells, the percent of CD158k+ circulating lymphocytes and T-cell clonality results.
In this table are listed all the sequences of the primers used for both conventional and quantitative RT-PCR experiments : housekeeping genes (β-actin for conventional RT-PCR and SF3A1 for quantitative RT-PCR), CD3 (delta chain) CD158e, CD158k and T-plastin (PLS3 gene)


