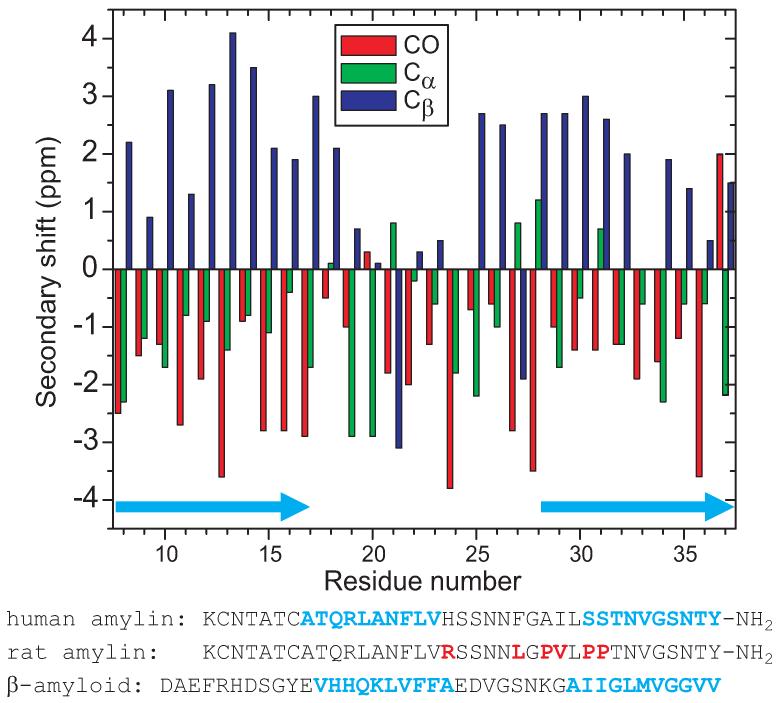
Figure 7.
13C NMR secondary chemical shifts for carbonyl, Cα, and Cβ sites in amylin fibrils with the morphology in Figs. 1C and 1D, determined from spectra in Figs. 4, 5, and 6. Light blue arrows indicate likely β-strand segments. Amino acid sequences of human amylin (with likely β-strands in light blue), rodent amylin (with amino acid differences in red), and β-amyloid (with previously determined β-strands (20-22) in light blue) are shown.