Abstract
The development of recombinant adeno-associated virus (rAAV) gene therapy applications is hampered by the inability to produce rAAV in sufficient quantities to support pre-clinical and clinical trials. Contrasting with adherent cell cultures, suspension cultures provide a straightforward means for expansion, however, transiently expressing the necessary, but cytotoxic virus proteins remains the challenge for rAAV production. Both the expansion and expression issues are resolved by using the baculovirus expression vector (BEV) and insect cell culture system. This review addresses strategies for the production of rAAV exploiting baculovirus technology at different scales using different configurations of bioreactors as well as processing and product characterization issues. The yields obtained with these optimized processes exceed ~1 × 1014 vector particles per liter of cell culture suitable for pre-clinical and clinical trials and possible commercialization.
Keywords: adeno-associated vectors, gene therapy, large-scale production, baculovirus, insect cell
Introduction
Adeno-associated virus, a dependovirinae genus member of the Parvoviridae Family are non-enveloped, icosahedral particles with linear, 4.7 kb single-strand DNA genome. The extraordinarily parsimonious genome contains two extensive open reading frames encoding the non-structural proteins required for replication (Rep) and the structural capsid proteins (VP). Three promoters regulate expression of the genes located at map positions (i.e. percent of the virus genome) 5 (p5), 19 (p19), and 40 (p40). The capsid consists of virus proteins 1, 2, and 3 (VP1, 2, and 3) in approximately 1:1:10 stoichiometry which also represents the expression levels in cells [reviewed in 1]. Although transcription from p40 produces a single transcript, the first in-frame methionine codon, used to initiate VP1 translation, is removed by splicing. In the spliced transcript, VP2 initiates inefficiently at ACG (threonine), however, the ribosomes continue scanning until encountering the first methionine codon that initiates translation of the most abundant capsid protein, VP3. The capsids assemble spontaneously from the soluble constituent proteins. The non-structural, Rep proteins, designated by the apparent molecular mass, Rep 78 and Rep 68 are expressed from p5, whereas expression of the Rep 52 and Rep 40 transcripts are regulated by the internal p19 promoter. Mostly the difference between Rep 78 and Rep 68, or Rep 52 and Rep 40, is attributable to splicing out of a short sequence near the 3′-end of the rep transcripts [reviewed in 1].
For AAV vector production in mammalian cell lines, the full-length open reading frames are typically provided with splicing determined by the producer cell. In addition to the AAV gene products, additional proteins from a helper virus are required for productive infection. As the name implies, the virus most commonly identified as an AAV helper virus is adenovirus, however, other DNA viruses may function as helper viruses.
Producing AAV vector requires the same set of virus proteins as does the wild-type virus. The linear, single-stranded DNA genome, unique to the Parvoviridae in the biological kingdom, replicates via a so-called “rolling hairpin” pathway initiating DNA synthesis at the 3′-terminus palindrome or inverted terminus repeat (ITR) [2]. The process is RNA-primer independent, and proceeds through leading strand DNA synthesis utilizing the host-cell DNA polymerase machinery. The intramolecular duplex replication intermediate is resolved into complementary DNA genomes through Rep-mediated terminal resolution [3]. The Rep 52 helicase activity is essential for packaging either of the complementary DNA strands into a capsid [4].
Recombinant AAV (rAAV) has several characteristics that are attractive for a gene transfer vector [reviewed in 5]. First, the wild-type virus is not considered pathogenic and is not known to cause human disease [6]. Second, the only genetic elements required in cis are the ITRs: no virus protein-coding genes are retained, thus limiting the host immune responses to the vector capsid proteins or the transgene protein product [7,8]. Third, rAAV DNA either remains episomal or inefficiently integrates into the host cell genome [9]. Fourth, in non-dividing cells, the vector DNA may remain transcriptionally active for long, often indeterminate lengths of time [10]. Fifth, rAAV can be produced from any of numerous capsid serotypes or isolates, which infect different cell types with various efficiencies or specificities [11]. Sixth, the particles are physically robust and withstand harsh treatment during processing [12,13]. Seventh, there have been no adverse events in clinical trials directly attributed to rAAV [14].
Undesirable characteristics of AAV vectors include: First, nearly universal prior exposure to one or more AAVs may result in circulating titers of neutralizing antibodies [15,16,17,18]. Second, relative inefficiency of transduction requires relatively large doses to obtain the desired effect. Third, the relatively slow onset of vector gene expression restricts applications. Fourth, the packaging capacity of the virus is essentially limited to the size of the wild-type virus DNA, 4.7 kb [19]. Fifth, importantly, rAAV is typically produced in adherent cells thereby limiting expansion for large doses, large clinical trials, or commercial production.
Different protocols based on AAV therapy have shown effectiveness in animal models to treat different genetic diseases including neurodegenerative diseases (e.g. Huntington’s disease [20,21,22]), solid cancer tumors [23], and ocular disorders (e.g. Leber’s congenital amaurosis, a cause of blindness [24]) among others. However, clinical trials for cystic fibrosis [25,26,27], and inherited hematological disorders (e.g. hemophilia B) [28] have produced disappointing results that were not predicted from the successful pre-clinical studies in rodents or dogs. Currently, clinical trials are being conducted for neurodegenerative diseases (e.g. Alzheimer’s [29,30], Canavan [31], and Parkinson’s diseases [32]), muscular diseases (e.g Duchenne muscular dystrophy [33]), metabolic disorders (e.g. hyperlipoproteinemia I [34]), and alpha-1-antitrypsin deficiency [35]), autoimmune diseases (e.g. rheumatoid arthritis [36]) among others.
A recently developed system for producing rAAV overcomes many of the constraints limiting rAAV production by utilizing baculovirus expression vectors (BEV) and the Sf9 cell line derived from lepidoptera Spodoptera frugiperda (Figure 1)[37]. Bevs are easily produced, characterized, and stored. Currently, Cervarix™ (GlaxoSmithKline) is the only insect cell - produced pharmaceutical product approved in Europe for human use. However, the papilloma vaccine consists of a virus-like particle, defined as the protein coat of the capsid without packaged nucleic acid [38]. In addition, clinical trials with insect cell-produced rAAV are underway for type I hyperlipoproteinemia, caused by lipoprotein lipase (LPL) deficiency (Amsterdam Molecular Therapeutics b.v.). The regulatory agencies’ acceptance of Sf9 produced biologicals and the commercialization potential of the system are additional benefits of producing rAAV in the Sf9-BEV system.
Figure 1.
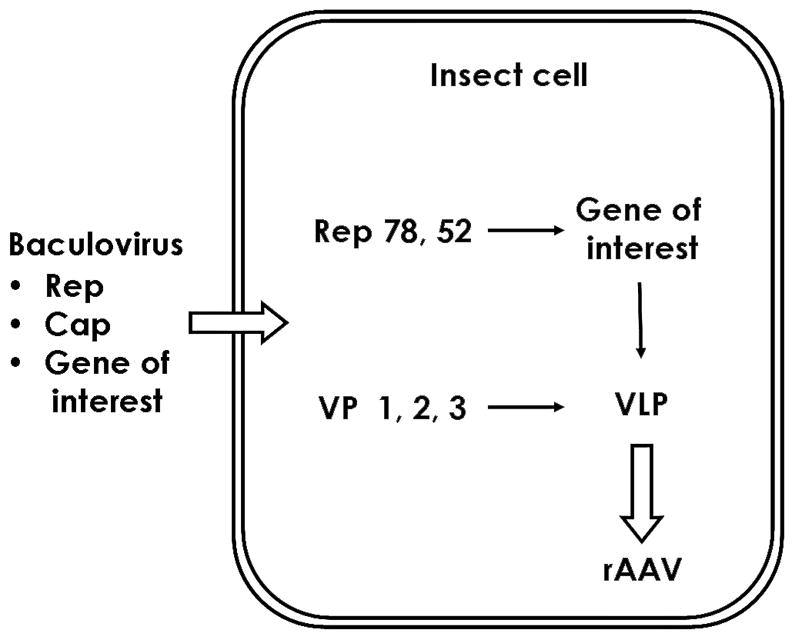
Schematic representation of rAAV production for gene therapy applications using the baculovirus-insect cell system. The insect cell is infected by three BEVs containing the rep, cap genes and the vector genome. After infection, the Rep 78 and Rep 52 proteins are expressed and the vector genome is rescued and replicated independently of the baculovirus DNA. Concurrently, the capsid proteins VP1, VP2, and VP3 are expressed and assembled into virus-like particles (VLP). The vector genome is packaged into the VLPs and producing the biologically active rAAV particle.
Producing rAAV at any scale using baculovirus – insect cell technology consists of four discrete phases: I) BEV production, II) rAAV production, III) rAAV recovery and purification, and IV) rAAV characterization (Figure 2).
Figure 2.
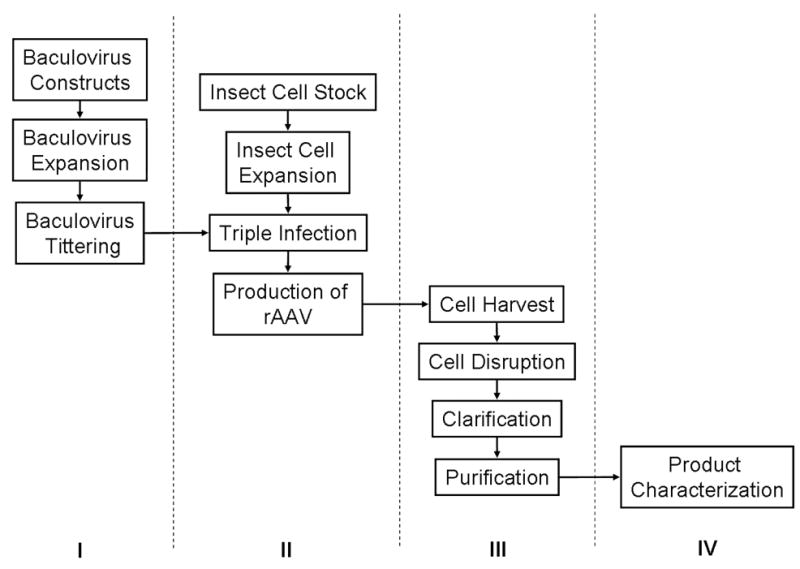
Phases for the production of rAAV in Sf9 cells – see text for details. I) Baculovirus production. II) rAAV production. III) rAAV purification. IV) rAAV characterization.
Bev production
Bevs are generated by homologous recombination in Sf9 insect cells co-transfected with a shuttle plasmid, e.g. pVL1392, and linearized baculovirus DNA that has a deletion of a vital gene (e.g. Baculogold, BD Biosciences, Inc.). Recombination with the homologous sequences of the shuttle plasmid provides the deleted vital gene in addition to the foreign DNA and produces an infectious baculovirus genome with the gene of interest. Alternative systems utilizing Escherichia coli harboring a “bacmid” provide a convenient means to rapidly generate infectious baculovirus genomes (FastBac, Invitrogen Inc.).
The number of cells and the multiplicity of infection (MOI) in the bioreactor determine the amount of BEVs required for rAAV production. As described above, the BEVs must provide the components for rAAV production: 1. genes that encode virus capsid proteins (VP1, VP2 and VP3), 2. genes that encode the replication protein (Rep 78 and Rep 52), and 3. AAV ITRs flanking the gene of interest (e.g., green fluorescent protein, or coagulation factor IX). As originally described, the BEVs were engineered to regulate cap expression at the translational level and rep expression at the transcriptional level to obtain optimal intracellular stoichiometries of the non-structural and structural proteins, respectively [37]. In the presence of the AAV Rep proteins, the rAAV vector DNA is “rescued” from the BEV genome and replicates as AAV independently of the baculovirus DNA apparently to very high copy numbers [37]. These replicated rAAV genomes are substrates for packaging into AAV capsids.
Expanding the amounts of each BEV clone is simply performed by serial passage in Sf9 cell culture infected at low MOI, e.g. 1:100 (v:v). After cell growth is arrested and viability decreases to about 20% the supernatant containing the baculovirus is harvested by centrifugation to remove cells and cell debris. Producing rAAV in Sf9 cells requires the simultaneous presence of the components. Since these cis and trans acting elements are provided as BEVs, accurately determining the BEV titer is important for obtaining high and reproducible rAAV yields. No rAAV can be generated in cells lacking any of the three BEVs. Plaque assays are considered the most consistent method for BEV titration. This bioassay provides a method to determine the infectious BEVs titer by analyzing serial dilutions of the original sample. In addition, a single plaque, representing a clonal BEV, may be selected for expansion. Plates containing plaques are useful for re-deriving essentially passage one BEV clones for additional or parallel expansion. Obtaining adequate amounts of the BEVs for a 1000 liter cell culture may require 3 expansion passages, depending on the MOI. The stability the BEVs is critical for maintaining consistency and reproducibility. Recent studies established that the BEVs constructs that contain the cap genes and the vector genome are stable for up to six passages whereas the originally described BEV with the rep genes are genetically less stable and allow expansion to passage four [39].
Recombinant AAV production
Small scale
Producing small amounts of rAAV for screening purposes or small experiments is most practical in disposable Erlenmeyer shake flasks, for example, polycarbonate vented screw-top flask (Corning Corp.). The temperature (27°C) and agitation (130 rpm) are maintained by a platform shaker incubator. Insect cells require relative high concentrations of dissolved oxygen (DO) typically around 30% for optimal cell growth and expression of BEV proteins. Although in the shake flask the DO is not monitored or controlled, the use of working volume not exceeding 30% of the total volume of the flask maintains the cell culture in exponential growth with high viability. The oxygen supply and gas exchange with the culture medium are facilitated with vented capped flasks. After cell growth is arrested and viability decreases to a value around 20% cell culture is harvested and processed for purification. The DO is normally correlated to product yield and in larger vessels the liquid – air interface is not adequate to sustain oxygen exchange [40,41]. Thus for larger volume cultures, alternative configurations that allow DO regulation are required.
Disposable bioreactors
Disposable, single-use bioreactors, such as the Wave™ system, are suitable for cell cultures ranging from 1L to 25L and provide a convenient option for mid-sized production of rAAV in insect cells [42]. Larger systems, up to 500L, are also available but have not been used for rAAV production. Using Wave™ bioreactors does not require strong bioprocessing background and the single-use sterile bags make the process significantly less complicated than reusable systems. A platform rocker with set points for rate and angle provides agitation, facilitates oxygenation, and maintains cells in suspension. The protocol for growing insect cells in this type of bioreactor is well documented [42,43,44]. Basically, the preparation for the process involves inflating the bag with sterile, filtered air followed by the addition of cell culture media. Once the media has reached the set temperature the bioreactor is inoculated with exponentially growing insect cells. Bag inflation is maintained by continuously supplying air or air/oxygen mixture to the head space. By adjusting the oxygen percentage in the gas mixture, the oxygenation of cell culture may be increased or decreased depending on growth conditions. In addition, other parameters may be monitored and controlled in this system including pH and temperature. The pH during insect cell growth and rAAV production is only monitored because this parameter does not change significantly during the process. Depending on the rAAV production scale, the density of insect cells may be expanded in situ thereby minimizing connections and potential contamination. For example, a 1L cell culture can be expanded to a 25L working volume with a single connection to the bioreactor. When cells duplicate and are in exponential phase fresh medium may be supplied until reaching the final working volume. Cells in exponential growth are then infected with baculovirus. Harvesting time is best determined by monitoring cell viability. Once the viable cell population falls below 30%, no discernible increase in rAAV yield occurs [45].
Stirred tank bioreactor
Operating stirred tank bioreactors (STBR) requires more complex infrastructure e.g. clean steam source and plumbing, as well as a larger capital investment for the equipment. Cleaning and sterilizing the STBR between runs with appropriate validation is laborious and time consuming. The STBR is normally used either as part of the process development or for the large scale manufacture of the product from hundreds to thousands of liters. The production of rAAV in STBR using the insect cells-baculovirus technology has been described in volumes ranging from 3L to 40L [42,45,46]. The main advantage of STBRs compared to Wave™ – type bioreactors is the ability to monitor and accurately control numerous parameters during the process. These controlled and on-line monitored parameters include temperature, agitation speed, DO, pH, and dielectric spectroscopy (or permittivity) for the production of rAAV [45]. The dielectric spectrum provides important information regarding the viable cell density and the cell growth rate. Events in the permittivity signal were correlated with simultaneous infection and the optimal harvesting time for rAAV [45]. One utility of STBR is that the production process is scalable and although the dimensions and proportions vary between vessels, e.g. the ratio of the cross sectional area to depth, the ability to monitor and control the environment provides the means to maintain uniform production independently of the culture volume. Similarly to Wave™ - type bioreactor, in the STBR cells can be expanded in situ from the minimum capacity of the bioreactor to the maximum working volume by adding fresh media when cells are growing exponentially. Once the final volume and cell density is reached, cells are infected with the BEVs. After analyzing manifold combinations of cell densities and MOIs, the optimal conditions for rAAV production in a STBR were determined to be a low MOI and low cell density at the time of infection (TOI). Because of the use of low MOI, cells duplicate before cell growth is arrested. This increases the number of producing cells and hence yields. Under these conditions, the cell culture can be harvested at viabilities greater than 60% without compromising rAAV quality and yield [39].
Recombinant AAV recovery and purification
One of the main challenges for manufacturing rAAV is recovering and purifying rAAV from cell components, media components, unassembled rAAV components, and baculovirus, without affecting the physical and biological integrity of the virus. Process optimization is critical to minimize rAAV losses at each stage of the downstream processing. The recovery and purification of rAAV from productions smaller than 1L are uncomplicated and high purity is achieved. At this scale, the downstream processing starts with the cell disruption and releasing the rAAV. A convenient method is three cycles of freezing and thawing at −80°C and 37°C, followed by centrifugation and vector recovery by polyethylene-glycol (2%, v:v) precipitation from the supernatant [39]. The precipitated material may be further processed by isopycnic gradient ultracentrifugation (e.g. cesium chloride). The buoyant densities of the empty and filled capsids are known (1.38g/cm3 and 1.41g/cm3, respectively; Figure 3).
Figure 3.
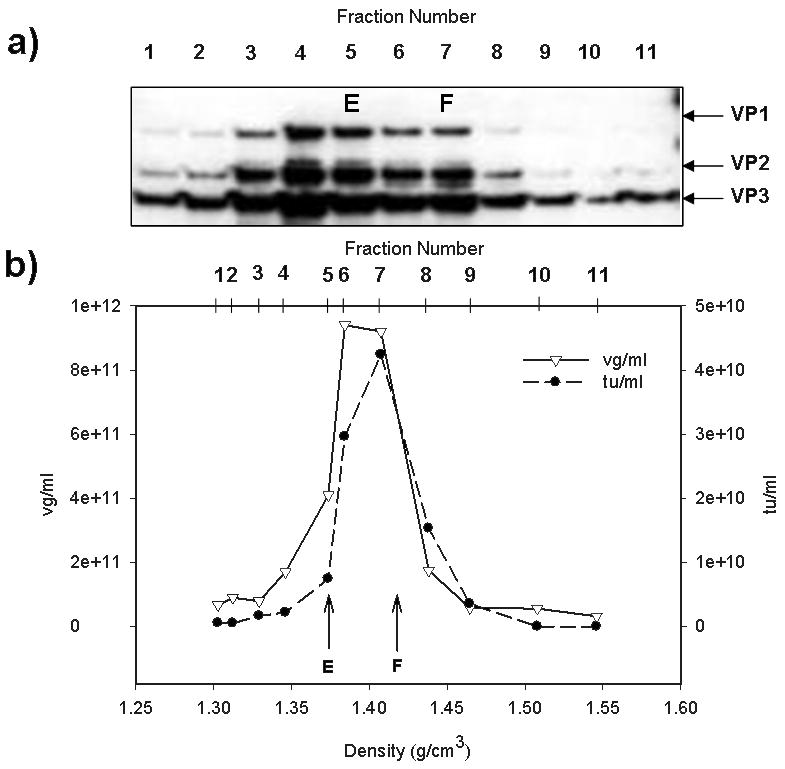
Characterization of rAAV using isopycnic CsCl gradient, DNA concentration, and biological activity. Gradient fractions (1 ml) were analyzed for: a) Presence of rAAV capsid proteins (VP1, VP2 and VP3) by polyacrylamide gel electrophoresis and western blot with anti-AAV capsid anti-serum and b) Concentration of vector genomes expressed as vector genomes (vg)/ml determined by qPCR. The biological activity or transduction units (tu)/ml determined by treating HEK 293 cells with dilutions of rAAV that express green fluorescent protein (GFP) and measuring the GFP positive cells using flow cytometry. The values of vg/ml and tu/ml are graphed for the CsCl isopycnic fractions. The peak activity values were obtained with densities corresponding to full capsids of rAAV (F). The position of the F and empty capsids (E) across the CsCl density gradient (1.41gcm3 and 1.37g/cm3, respectively) are indicated. The corresponding fraction numbers are indicated in each panel.
Large scale purification of rAAV
The transition from small- to large- scale purification of rAAV requires developing processes appropriate for the larger volumes. Although it is possible to recover intracellular rAAV by separating cells from the medium by cross-flow or tangential flow filtration, processing the entire biomass and cell culture volume enables recovery of both intracellular and extracellular rAAV. For large volumes, disrupting cells by freezing and thawing are impractical whereas either chemical or mechanical methods are acceptable. Chemical disruption using non-ionic surfactants is a convenient and inexpensive method. However, removing these compounds during the purification increases the number of processing steps, validation assays, and cost, which compromise the net yield of rAAV. Mechanically disrupting cells is consistent, reproducible, and may produce more recoverable rAAV. The disadvantages include the high initial cost of pilot-scale homogenizers, large footprint, and validation assays for cleaning and operating the instrument.
After disrupting the cells it is necessary to separate the rAAV particles from the cell debris. Filtration provides convenient formats for clarification, concentration, and even buffer exchange (diafiltration). The two most popular modes of filtration are dead-end and tangential flow filtration (TFF). Within each system numerous filter compositions and configurations available. Empirically determining the appropriate filtration system is perhaps the only reliable method for assessing filters. The characteristics of filter membranes, even of identical nominal molecular weight cut off (MWCO) differ between manufacturers. In addition, there are many permutations of available filter configurations. Dead-end filters are available as sterile single-use, disposable capsules that are compatible with cGMP processing. However, one of the limitations is the capacity of the filter that is related to the filtration surface area. The rAAV recovered in the filtrate diminishes as the capacity of the filter capsule is approached which is determined experimentally. Replacing the filter capsule prevents loss of rAAV through trapping in the filter membrane.
Both dead-end and TFF systems have overlapping applications, e.g. clarification, however, there are additional processes that are specific for TFF. Buffer exchange, or diafiltration, is a commonly used process intended for the rAAV to remain in the retentate and allows for vector concentration, and some removal of impurities. However, a TFF system suitable for processing hundreds or thousands of liters involves a large capital investment, as well as cleaning and sanitizing validation steps.
Recovering rAAV from the clarified, filtered, and concentrated material by column chromatography is probably the most versatile and scalable process and can be used under cGMP compliance. Different protocols are available using ion exchange chromatography for purification of different serotypes of rAAV [12,47,48,49,50,51]. Ion exchange chromatography medium interacts with the surface charges of the rAAV particle which are eluted by increasing the ionic strength of column buffer. In a further refinement, the separation of full capsids from empty capsids was described although it is uncertain whether this process can be readily scaled-up [52,53]. Additionally, heparin-affinity chromatography has successfully purified rAAV serotype 2 efficiently [54]. A recently developed immunoaffinity resin is now commercially available. The efficiency of capturing the rAAV particle with the single-chain, recombinant antibody depends primarily on the flow rate. Highly purified preparations of rAAV are obtained after the chromatographic step (Figure 4). This is an easily scalable process, simply increasing the column diameter and the amount of resin to maintain the resin bed height produces equivalent results for any scale process. The chromatography media may be regenerated and sanitized for further use.
Figure 4.
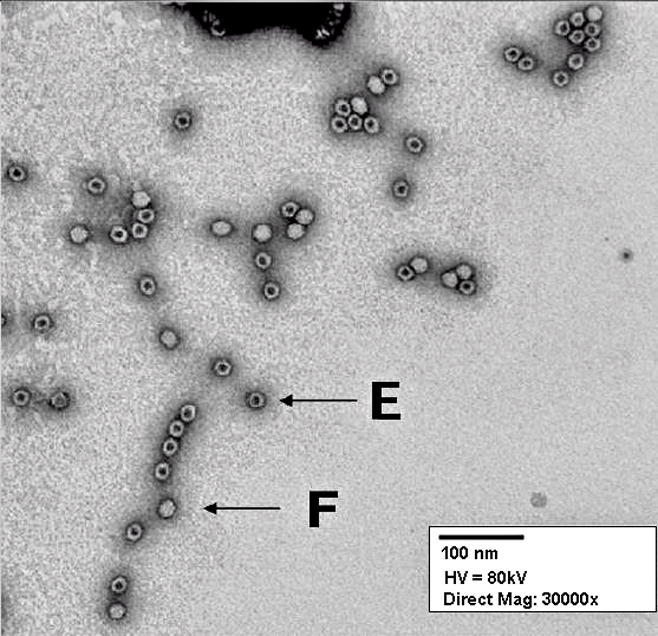
Electron microscopic visualization of rAAV full capsids (F) and empty capsids (E) after the affinity column chromatography purification step by electron microscopy. 30000X magnification.
Product characterization
The rAAV particle is a simple nucleoprotein complex consisting of three structural proteins and DNA. Determining the composition of the rAAV during production and the final preparations involves polyacrylamide gel electrophoresis (PAGE) and sensitive protein staining, usually silver stain. PAGE and silver staining are used to provide an estimate of the ratio of the capsid proteins VP1, VP2 and VP3, and to assess the relative purity of the capsid proteins in the final preparation.
Following nuclease treatment to degrade non-encapsidated DNA, the vector genome (DNA) copy number can be determined either by quantitative polymerase chain reaction (qPCR), hybridization of vector DNA to a vector specific labeled probe, quantitative Southern blot, or spectrophotometrically.
Following infection, samples obtained from the bioreactor at various intervals and processed as described above are useful for determining the time-course of rAAV production and harvest time. Based on analysis of bioreactor rAAV production, the detectable capsid components of rAAV increase over the time, and remain constant or increase until the cell viability falls below 30%. It is possible to visualize the distribution of capsid proteins across the CsCl gradient on western blots using anti-capsid antibody (Figure 3a). The intensity of the bands corresponding to VP1, VP2 and VP3 in the western blot analysis is enhanced at densities where full and empty capsids are expected.
The vector genome concentration corresponds to the number of full capsids or the number of vector genomes encapsidated into DNAse resistant particles (Figure 3b). For example, using qPCR, the concentration or copy number of the vector genome may be determined relative to a set of standards. The result obtained from the qPCR is commonly used to evaluate the success of the production process and establish reproducibility between production runs.
Vector biological activity
The bioactivity of the produced rAAV is crucial for gene therapy applications. Testing in cell lines is commonly used to assess the rAAV bioactivity: cell lines such as human embryonic kidney 293 cells are easily maintained and are well characterized (Figure 3b). However, often the effect of the vector is specific to a cell type and cannot be measured in established cell lines, making the evaluation of biological activity difficult. The potency of the vector is determined by the activity per unit, e.g., 1 transducing unit per 1000 vector genome-containing particles. The reason for the relatively large doses required in vivo, for LPL clinical trials, doses of 2 × 1012 vector genome-containing particles per kg were used, is not fully determined, and is probably attributable to several effects involving binding and uptake efficiency, multiple vector genomes required per cell, neutralizing antibodies, excretion, and retention in the liver.
Additionally, for more complete characterization analysis, the total number of rAAV particles produced can be obtained. This titer can be determined by immuno-detection assays (e.g. ELISA). Kits for immuno-detection and quantification of different serotypes of rAAV are commercially available. Also, the ratio of absorbance at 280nm and 260nm in pure rAAV samples can be used as part of the characterization analysis. The results of these analyses become more significant when they are supported by other titration assays.
Conclusions
The reproducible production of large amounts of high quality rAAV is possible at different scales. This can be performed using different configuration of bioreactors including shake-flasks, Wave™ -type bioreactors and STBRs. Depending on the requirements of the projects, the production of rAAV exploiting insect cell technology provides sufficient material to perform numerous experiments in vitro and in vivo in animal models. High purity of rAAV can be achieved at different scales by using the appropriate processing operations. The selection for downstream processing operations depends on the volume of material to be processed, equipment available, and cGMP compliance requirements. Several assays are combined for accurate characterization of the final product. Considering all conditions mentioned, the production of rAAV for gene therapy applications can be produced in about any laboratory at different scales.
Key Points
Large amounts of high quality rAAV can be produced at different scales independently of bioreactor configuration and infrastructure of facilities.
Typical yields of ~1e14 vector genomes per liter of rAAV are obtained using the baculovirus-insect cell technology.
The reproducibility of bioactive rAAV titer obtained among runs strongly depends on I) quality of BEVs, II) Process conditions during production, III) Type of technology selected for recovery and purification and IV) product scrutiny based on combined characterization analysis.
Acknowledgments
The Division of Intramural Research, National Heart, Lung, and Blood Institute of the National Institutes of Health provided funding for this research. The Parent Project Duchenne Muscular Dystrophy, International Collaborative Effort for Duchenne Muscular Dystrophy, and a Cooperative Research and Development Agreement (CRADA) with Amsterdam Molecular Therapeutics provided additional funds.
References
- 1.Smith RH, Kotin MR. Adeno-associated virus. In: Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. ASM Press; Washington, D.C: 2002. pp. 905–923. [Google Scholar]
- 2.Tattersall P, Ward DC. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976;263:106–9. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- 3.Im DS, Muzyczka N. Partial purification of adeno-associated virus Rep78, Rep52, and Rep40 and their biochemical characterization. J Virol. 1992;66:1119–28. doi: 10.1128/jvi.66.2.1119-1128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chejanovsky N, Carter BJ. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology. 1989;173:120–8. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 5.Kotin RM. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 6.Monahan PE, Jooss K, Sands MS. Safety of adeno-associated virus gene therapy vectors: a current evaluation. Expert Opin Drug Saf. 2002;1:79–91. doi: 10.1517/14740338.1.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984;81:6466–70. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–28. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–45. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 10.Ehrhardt A, Yant SR, Giering JC, et al. Somatic integration from an adenoviral hybrid vector into a hot spot in mouse liver results in persistent transgene expression levels in vivo. Mol Ther. 2007;15:146–56. doi: 10.1038/sj.mt.6300011. [DOI] [PubMed] [Google Scholar]
- 11.Grimm D, Kay MA, Kleinschmidt JA. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther. 2003;7:839–50. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith RH, Ding C, Kotin RM. Serum-free production and column purification of adeno-associated virus type 5. J Virol Methods. 2003;114:115–24. doi: 10.1016/j.jviromet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Anderson R, Macdonald I, Corbett T, et al. A method for the preparation of highly purified adeno-associated virus using affinity column chromatography, protease digestion and solvent extraction. J Virol Methods. 2000;85:23–34. doi: 10.1016/s0166-0934(99)00150-0. [DOI] [PubMed] [Google Scholar]
- 14.Flotte TR. Gene therapy: the first two decades and the current state-of-the-Art. J Cell Physiol. 2007;213:301–5. doi: 10.1002/jcp.21173. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SL, Li H, Zhou S, Schlachterman A, High K. Prolonged Susceptibility to Antibody-mediated Neutralization for Adeno-associated Vectors Targeted to the Liver. Mol Ther. 2008;16:138–45. doi: 10.1038/sj.mt.6300334. [DOI] [PubMed] [Google Scholar]
- 16.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–17. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 17.Halbert CL, Miller AD, McNamara S, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–47. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peden CS, Burger C, Muzyczka N, et al. RJ Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–59. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–12. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 20.Harper SQ, Staber PD, He X, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubansu A, Abeloos L, Bockstael O, et al. Recombinant AAV viral vectors serotype 1, 2, and 5 mediate differential gene transfer efficiency in rat striatal fetal grafts. Cell Transplant. 2008;16:1013–20. [PubMed] [Google Scholar]
- 22.Xia H, Mao Q, Eliason SL, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–20. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Yang L, Scudiero DA, et al. Development of recombinant adeno-associated virus vectors carrying small interfering RNA (shHec1)-mediated depletion of kinetochore Hec1 protein in tumor cells. Gene Ther. 2007;14:814–27. doi: 10.1038/sj.gt.3302933. [DOI] [PubMed] [Google Scholar]
- 24.Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–65. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aitken ML, Moss RB, Waltz DA, et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther. 2001;12:1907–16. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- 26.Flotte TR. Recent developments in recombinant AAV-mediated gene therapy for lung diseases. Curr Gene Ther. 2005;5:361–66. doi: 10.2174/1566523054064986. [DOI] [PubMed] [Google Scholar]
- 27.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–98. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manno CS, Arruda VR, Pierce GF, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–47. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 29.Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–50. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 30.Fukuchi KI, Tahara K, Kim HD, et al. Anti-Abeta single chain antibody delivery via adeno-associated virus for treatment of Alzheimer’s disease. Neurobiol Dis. 2006;23:502–11. doi: 10.1016/j.nbd.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPhee SW, Janson CG, Li C, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–88. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- 32.Fiandaca M, Forsayeth J, Bankiewicz K. Current status of gene therapy trials for Parkinson’s disease. Exp Neurology. 2008;209:51–7. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Muntoni F, Wells D. Genetic treatments in muscular dystrophies. Curr Opin Neurol. 2007;20:590–4. doi: 10.1097/WCO.0b013e3282efc157. [DOI] [PubMed] [Google Scholar]
- 34.Rip J, Nierman MC, Sierts JA, et al. Gene therapy for lipoprotein lipase deficiency: working toward clinical application. Hum Gene Ther. 2005;16:1276–86. doi: 10.1089/hum.2005.16.1276. [DOI] [PubMed] [Google Scholar]
- 35.Brantly ML, Spencer LT, Humphries M, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17:1177–86. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- 36.Boissier MC, Lemeiter D, Clavel C, et al. Synoviocyte infection with adeno-associated virus (AAV) is neutralized by human synovial fluid from arthritis patients and depends on AAV serotype. Hum Gene Ther. 2007;18:525–35. doi: 10.1089/hum.2006.174. [DOI] [PubMed] [Google Scholar]
- 37.Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–43. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 38.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 39.Negrete A, Yang LC, Mendez AF, et al. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J Gene Med. 2007;9:938–48. doi: 10.1002/jgm.1092. [DOI] [PubMed] [Google Scholar]
- 40.Saarinen MA, Murhammer DW. The response of virally infected insect cells to dissolved oxygen concentration: Recombinant protein production and oxidative damage. Biotech Bioeng. 2002;81:106–14. doi: 10.1002/bit.10460. [DOI] [PubMed] [Google Scholar]
- 41.Gotoh T, Chiba K, Kikuchi KI. Oxygen consumption profiles of SF-9 insect cells and their culture at low temperature to circumvent oxygen starvation. Biochem Eng J. 2004;17:71–8. [Google Scholar]
- 42.Negrete A, Kotin RM. Production of recombinant adeno-associated vectors using two bioreactor configurations at different scales. J Virol Methods. 2007;145:155–61. doi: 10.1016/j.jviromet.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadwell SH, Hardwicke PI. Production of baculovirus-expressed recombinant proteins in wave bioreactors. Methods Mol Biol. 2007;388:247–63. doi: 10.1007/978-1-59745-457-5_12. [DOI] [PubMed] [Google Scholar]
- 44.Cronin CN, Lim KB, Rogers J. Production of selenomethionyl-derivatized proteins in baculovirus-infected insect cells. Protein Sci. 2007;16:2023–9. doi: 10.1110/ps.072931407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negrete A, Esteban G, Kotin RM. Process optimization of large-scale production of recombinant adeno-associated vectors using dielectric spectroscopy. Appl Microbiol Biotechnol. 2007;76:761–72. doi: 10.1007/s00253-007-1030-9. [DOI] [PubMed] [Google Scholar]
- 46.Meghrous J, Aucoin MG, Jacob D, et al. Production of recombinant adeno-associated viral vectors using a baculovirus/insect cell suspension culture system: from shake flasks to a 20-L bioreactor. Biotechnol Prog. 2005;21:154–60. doi: 10.1021/bp049802e. [DOI] [PubMed] [Google Scholar]
- 47.Gao G, Qu G, Burnham MS, et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–91. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- 48.Brument N, Morenweiser R, Blouin V, et al. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol Ther. 2002;6:678–86. doi: 10.1006/mthe.2002.0719. [DOI] [PubMed] [Google Scholar]
- 49.Kaludov N, Handelman B, Chiorini JA. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum Gene Ther. 2002;13:1235–43. doi: 10.1089/104303402320139014. [DOI] [PubMed] [Google Scholar]
- 50.Zolotukhin S, Potter M, Zolotukhin I, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–67. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 51.Davidoff AM, Ng CY, Sleep S, et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J Virol Methods. 2004;121:209–15. doi: 10.1016/j.jviromet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Urabe M, Xin KQ, Obara Y, et al. Removal of empty capsids from type 1 adeno-associated virus vector stocks by anion-exchange chromatography potentiates transgene expression. Mol Ther. 2006;13:823–8. doi: 10.1016/j.ymthe.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Qu G, Bahr-Davidson J, Prado J, et al. Separation of adeno-associated virus type 2 empty particles from genome containing vector by anion-exchange column chromatography. J Virol Meth. 2007;140:183–92. doi: 10.1016/j.jviromet.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Auricchio A, Hildinger M, O’Connor E, et al. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12:71–6. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]


