Figure 5. Drawing analogies between tau and receptor tyrosine kinases.
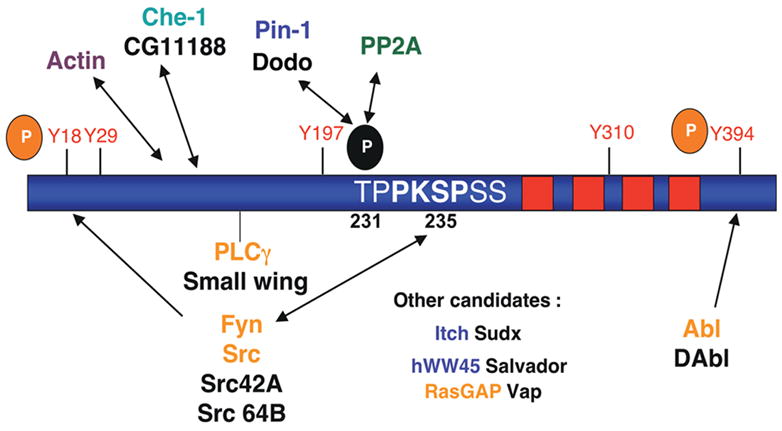
Tau contains 14 SP/TP, 5 tyrosines (at least two of which - Y18 and Y394 - are known to be phosphorylated in PHF-tau), and 7 PXXP-type motifs, making it a potential binding partner for WW, SH2 and SH3 domain-containing proteins, respectively. In cell culture and in vitro systems, tau is known to bind to SH2/3-containing proteins including Src family non-receptor tyrosine kinases [51], c-Abl [90] and PLC-γ [91, 92]. Binding of tau to the Fyn-SH3 domain occurs at the PXPXXP sequence shown (232-236). Tau also binds the WW domain-containing protein Pin-1, which isomerizes P-Thr-231 to render it more readily dephosphorylated by PP2A [93]. Other candidate proteins of interest, with genetic reagents available in flies, include the WW domain-containing proteins hWW-45 and Itch and the SH2/SH3-containing RASGAP. Note that fly homologs are labeled in black.
