Figure 6. TOR signaling in Drosophila [94–96].
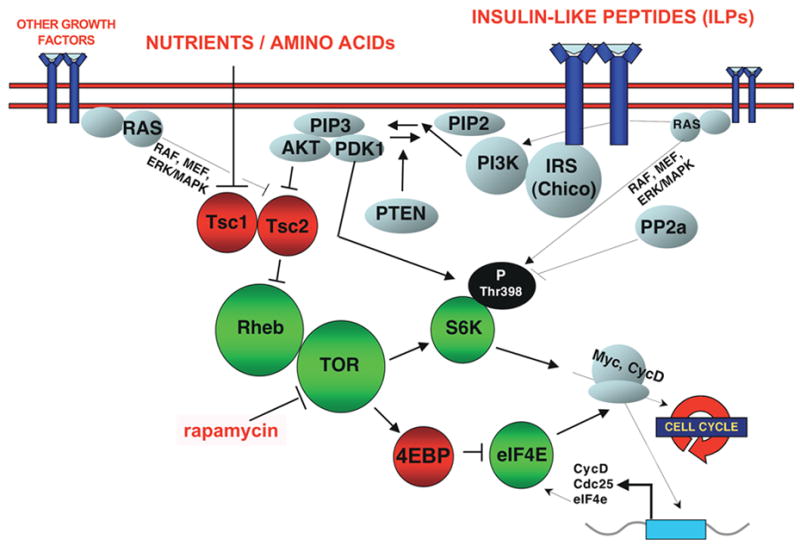
TOR integrates nutrient availability with signals from multiple pathways, including insulin signaling/PI3K. The insulin receptor is a ~400kDa tyrosine kinase receptor. PI3K is activated by direct recruitment to the insulin receptor substrate (IRS; also known as Chico). In mammalian systems, a well-characterized mechanism of PI3K activation is through Ras. PI3K appears to activate MAP kinase pathways in mammalian cells, but this does not appear to be the case in flies [96]. However, signaling from PI3K to Akt, and to TOR is well conserved. Akt phosphorylates and inhibits Tsc2 [97], the GAP protein inhibitory for the Rheb GTPase [98]. Rheb activates TOR and drives growth and indirectly the G1/S transition [99–101]. Over-expressing TOR itself paradoxically downregulates TOR signaling [102]. The two major downstream effectors are S6k and eIF4e. In flies, TOR activates S6k by phosphorylating Thr398. eIF4E is inhibited by 4EBP and TOR-dependent phosphorylation of 4EBP relieves this inhibition, leading to protein translation [103]. Thin arrows indicate links established in other systems. Studies in mammalian cell-culture systems have demonstrated that S6K phosphorylates the ribosomal protein S6 and thus activates ribosome biogenesis. eIF4E activates Cap-dependent protein translation. Targets may include Cyclin D and the Myc oncogene [61]. eIF4e is in turn a transcriptional target of Myc [104].
