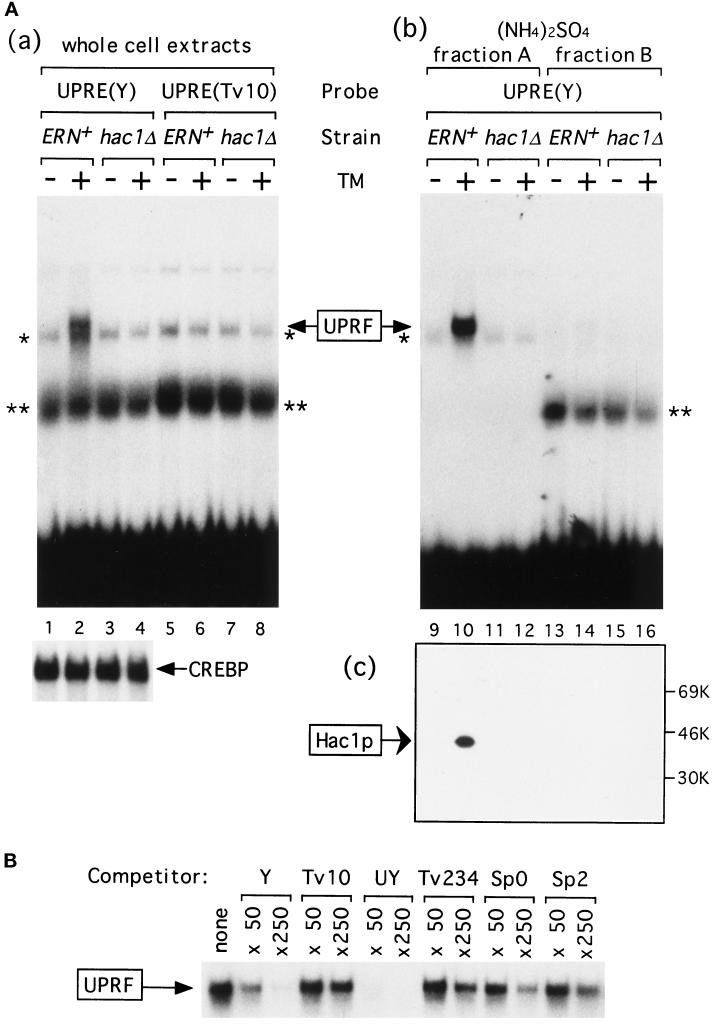
Figure 1.
Induction of Hac1p by tunicamycin. (A) Whole cell extracts, (NH4)2SO4 fraction A, and (NH4)2SO4 fraction B were prepared from the ERN+ (KMY1105) and hac1Δ (KMY1145) strains that had been grown in YPD medium to a midlogarithmic phase and incubated for 1 h with (+) or without (−) tunicamycin (TM). (a) Forty micrograms of proteins in each of whole cell extracts were mixed with 0.3 ng of 32P-labeled wild-type UPRE [designated UPRE(Y), 9060 cpm], a point mutant of UPRE [designated UPRE(Tv10), 9390 cpm], or CRE (7590 cpm). (b) Forty micrograms of proteins in each of (NH4)2SO4 fraction A or fraction B were mixed with 0.2 ng (9130 cpm) of 32P-labeled UPRE(Y). Protein-bound probes were separated from free probes in a 5% nondenaturing gel. The specific binding to UPRE(Y) is marked as UPRF. * and ** denote nonspecific bindings that were also detected when 32P-labeled CRE was used as a probe. Only specific binding to CRE was shown below and marked as CREBP. (c) Fifty micrograms of proteins in (NH4)2SO4 fraction A or fraction B were subjected to SDS-PAGE (12% gel) and immunoblotted with purified anti-Hac1p antibodies. The positions of molecular mass markers are indicated. (B) The specific binding between 0.3 ng (7870 cpm) of 32P-labeled UPRE(Y) and UPRF in the (NH4)2SO4 fraction A (20 μg of proteins) from tunicamycin-treated ERN+ strain was competed by 50- or 250-fold molar excess of unlabeled UPRE(Y) or various mutant forms of UPRE, whose activity in mediating the UPR was well characterized previously (Mori et al., 1996). Only specific binding is shown. Tv10, a point mutant of Y described in the text, is virtually inactive in mediating the UPR in vivo. UY contains a palindrome of 7 bp that is more active than Y. Tv234 contains three transversions upstream of the palindromic sequence. Sp0 and Sp2 are mutant versions of UPRE in which the half-sites in the palindromic sequence are separated by spacers of 0 and 2 nt, respectively, instead of 1 nt in Y. Tv234, Sp0, and Sp2 exhibit very weak activities in vivo. Neither 5′ nor 3′ termini of competitor oligonucleotides were filled in, and possible formation of concatemers may explain the large molar excess of unlabeled UPRE-Y required for competition under these conditions.