Figure 2. Four-jointed dependent phosphorylation of Ds and Fat cadherin domains.
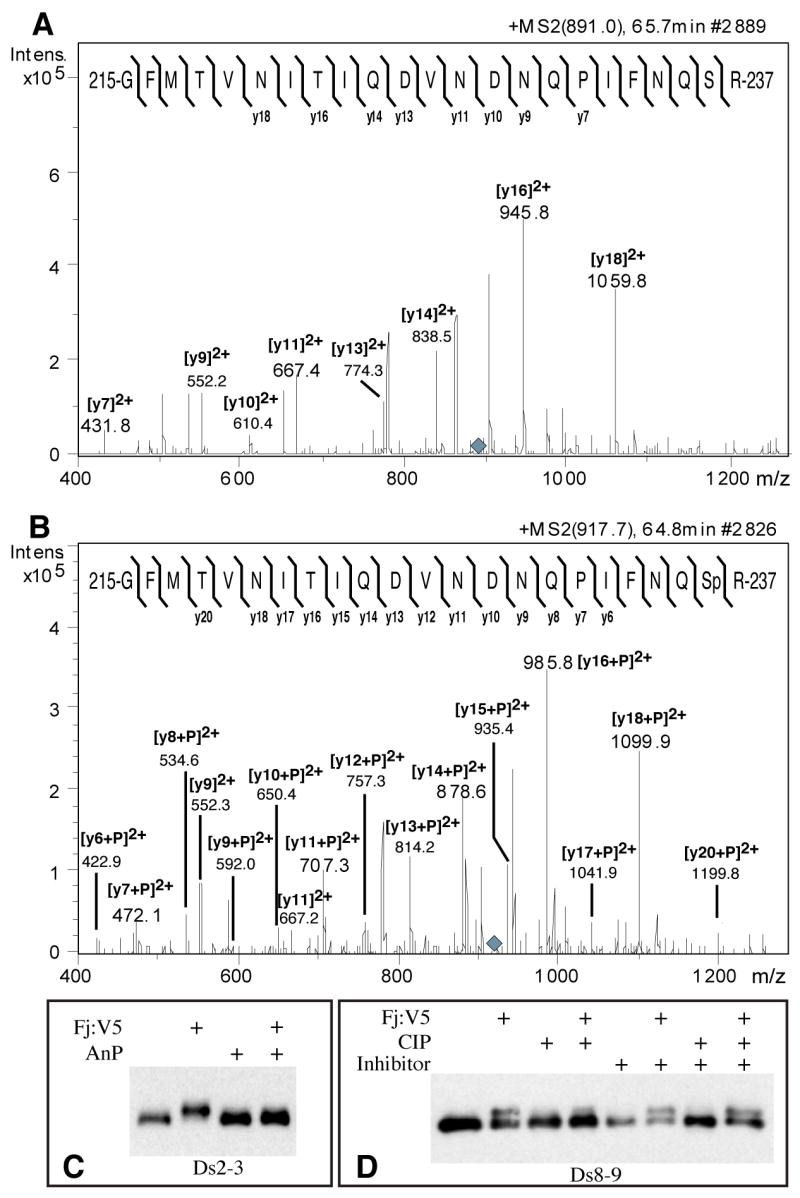
A) MS/MS fragmentation of a tryptic peptide from Ds2-3:FLAG isolated from S2 cells, eluting on HPLC at 65.7 minutes (Supplementary Fig. S3A). The parental ion is triply charged (m/z 891.0, grey diamond). The mass and fragmentation pattern identify this as aa 215-237; the positions of identified y ions from peptide fragmentation are indicated. B) MS/MS fragmentation of a tryptic peptide from Ds2-3:FLAG isolated from Fj:V5-expressing S2 cells, eluting on HPLC at 64.8 minutes (parent ion m/z 917.7, grey diamond). The positions of identified y ions from peptide fragmentation are indicated. C) Western blot on Ds2-3:FLAG isolated from S2 cell media, indicated cells expressed Fj:V5, and/or were treated with antarctic phosphatase (AnP). D) Western blot on Ds8-9:FLAG isolated from S2 cell media, indicated cells expressed Fj:V5, and/or treated with calf intestinal alkaline phosphatase (CIP), and/or treated with phosphatase inhibitor cocktail.
