Fig. 5.
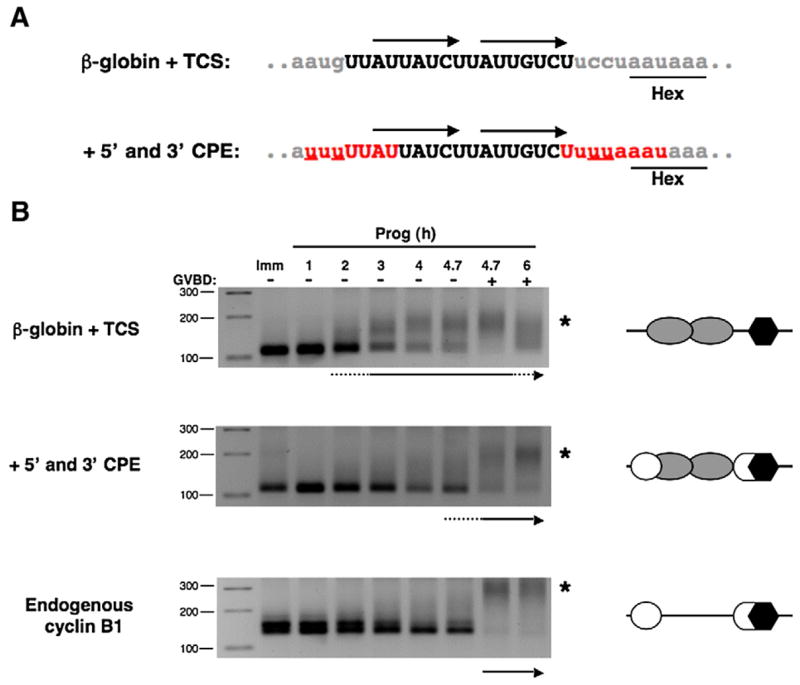
The presence of flanking CPE sequences suppresses TCS function. (A) Sequence and regulatory element composition of β-globin 3′ UTR reporter constructs employed. The β-globin 3′ UTR containing the two TCS elements (upper panel, arrows) was mutated (underlined red nucleotides) to generate 5′ and 3′ CPE sequences found in the wild-type Wee1 3′ UTR (lower panel). Note that the 3′ CPE overlaps the polyadenylation hexanucleotide. Lower case gray letters represent β-globin sequence, upper case black nucleotides represent Wee1 sequence, and red nucleotides represent reconstituted CPE sequences. (B) Immature oocytes were separately injected with GST reporter mRNAs fused to the β-globin UTR containing a Wee1 TCS (β-globin+TCS) or the Wee1 TCS flanked with both a 5′ CPE and 3′ hexanucleotide overlapping CPE (+5′ and 3′ CPE) that replicate the sequence and position of the CPE sequences within the endogenous Wee1 3′ UTR. RNA samples were taken from progesterone-stimulated oocytes at the indicated times and the polyadenylation of the reporter mRNAs and the endogenous cyclin B1 mRNA was assessed by RNA ligation PCR. Reporter RNA polyadenylation was assessed using a forward primer specific to the β-globin 3′ UTR of the injected reporter mRNAs. A schematic of the regulatory element arrangement within the 3′ UTRs is depicted beside each polyadenylation assay, where open circles represent CPEs, gray ovals are TCS elements, and polyadenylation hexanucleotides are shown as black hexagons. The initiation of polyadenylation for each mRNA examined is indicated by an arrow beneath each panel and the extent of GVBD indicated above the top panel. GVBD50 occurred after 4.7 h of stimulation. An asterisk indicates the position of polyadenylated mRNA species.
