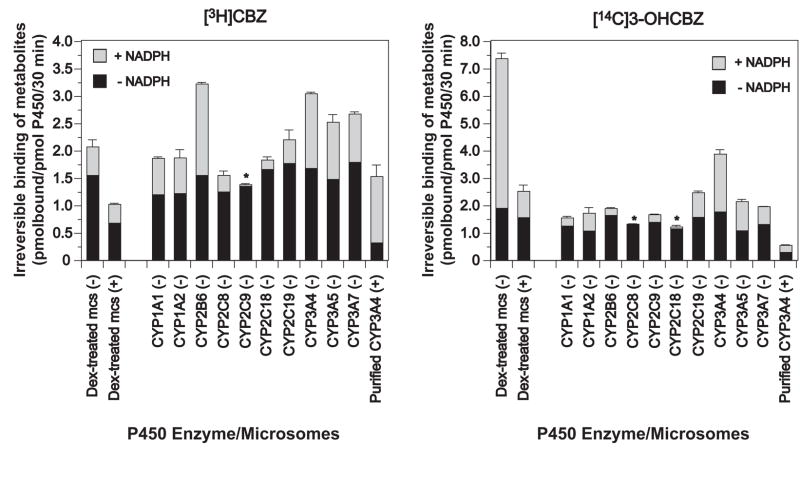
Figure 12.
Irreversible binding of metabolites generated from [3H]CBZ or [14C]3-OHCBZ to mouse liver microsomal or human recombinant P450 proteins. CYP3A enriched liver microsomes from dexamethasone-treated (Dex-treated) mice or microsomes containing cDNA-expressed human P450 enzymes co-expressed with P450 reductase and cytochrome b5 (BD Gentest Supersomes™) were incubated with [3H]CBZ or [14C]3-OHCBZ (0.2 μCi; 0.5 mM) in the presence or absence of NADPH (+ NADPH and − NADPH, respectively), as described in Materials and Methods. Incubations conducted in the absence of NADPH were performed in duplicate, whereas incubations conducted in the presence of NADPH were performed at least in triplicate (N = 3 or 4). Some incubations also contained freshly prepared GSH (1 mM or 4mM in incubations containing liver microsomes from Dex-treated mice or purified recombinant CYP3A4 functionally reconstituted, respectively) and are denoted with a (+); whereas incubations conducted in the absence of GSH are denoted with a (−). Functional reconstitution of purified CYP3A4 requires that GSH be included for optimal activity (Gillam et al, 1993), hence incubations containing purified CYP3A4 in the absence of GSH were not performed. After 30 min, reactions were terminated with ice-cold methanol/5% H2SO4, microsomal proteins precipitated, sedimented and sequentially “washed” to reduce non-specifically bound radiolabelled substrates or metabolites, as described in Materials and Methods. Each of the rates of irreversible binding determined in incubations performed in the presence of NADPH were found to be significantly different from the corresponding rates determined in incubations performed in the absence of NADPH (controls) as detemined using a two-tailed, paired Student’s t test (p ≤ 0.05). The incubations denoted with an asterisk (*) were found to be not statistically different.