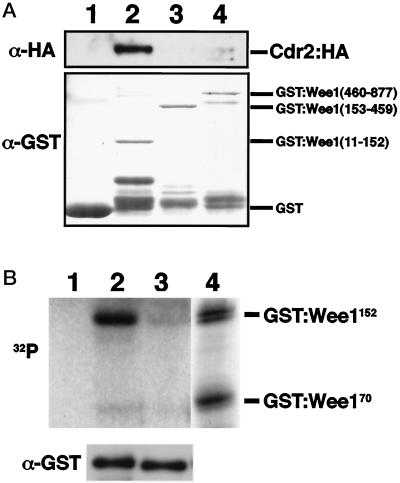
Figure 10.
Cdr2 binds and phosphorylates the N-terminus of Wee1 in vitro. (A) Lysates from cells expressing HA epitope-tagged Cdr2 were incubated with GST or GST–Wee1 proteins purified from bacteria with GSH–Sepharose. After extensive washing, samples were subjected to immunoblotting with anti-HA or anti-GST antibodies. Lane 1, GST; lane 2, GST–Wee1(11–152); lane 3, GST–Wee1(153–459); lane 4, GST–Wee1(460–877). (B) GST–Cdr2, GST–Cdr2K39A, and GST–Cds1 proteins were prepared from fission yeast and bound to GSH–Sepharose. Each protein was mixed with GST–Wee1(11–152) protein bound to GSH–Sepharose and incubated in a kinase reaction. Samples were resolved by 12% SDS-PAGE. Lane 1, GST–Wee1 only; lane 2, GST–Cdr2; lane 3, GST–Cdr2K39A; lane 4, GST–Cds1. An anti-GST blotting shows that almost equal amounts of GST–Cdr2 and –Cdr2K39A proteins were used in lane 2 and in lane 3. Note that a mobility shift of GST–Wee1(11–152) caused by phosphorylation by Cds1 was detected in lane 4.