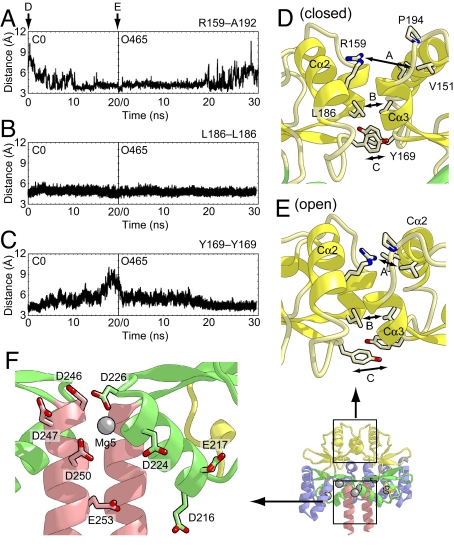
Fig. 5.
The interactions between the two CBS domains that enable the domain motion upon Mg2+ binding. (A) The distances between the Cζ atom of Arg-159 in one subunit and the main-chain carbonyl atom of Ala-192 in another subunit as a function of time during the O5 and O465 simulations. (B and C) The distances between the Cγ atoms of Leu-186 (B) and Tyr-169 (C) in both subunits during the simulations. (D and E) Close-up views of the subunit interface of CBS1 in the initial structures of the C0 (D) and O465 (E) simulations. The protein side chains discussed in the text are shown as stick models. The distances shown in A–C are indicated. (F) Close-up view of the subunit interface of CBS2 in the Mg2+-bound crystal structure. The protein side chains of acidic residues are shown as stick models.