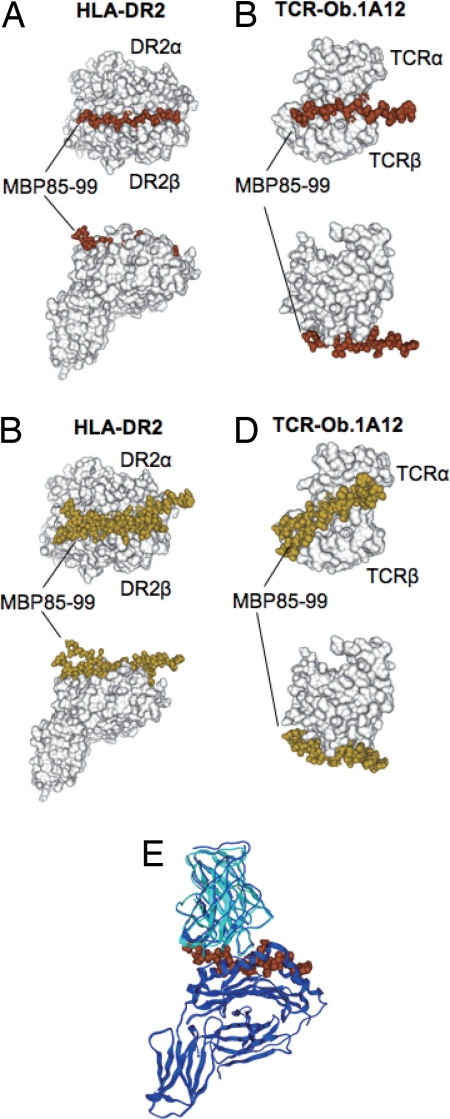
Fig. 2.
Docking simulation of the MBP85–99 peptide on HLA-DR2 and on TCR-Ob.1A12. (A) The four clustered docked peptides of MBP85–99 on HLA-DR2 are indicated as space filling models in brown. (B) The six nonclustered docked peptides are indicated as space filling models in yellow. In A and B, HLA-DR2 (DRB1*1501/DRA) (10) is shown as a surface model in white. (C) The six clustered docked peptides of MBP85–99 on TCR-Ob.1A12 are indicated as space filling models in brown. (D) The four nonclustered docked peptides are indicated as space filling models in yellow. In C and D, TCR-Ob.1A12 is shown as a surface model in white. (E) Merging of the docked TCR-Ob.1A12/MBP85–99 and MBP85–99/HLA-DR2 was carried out by using the conformation of the peptide as the basis for merging. Superposition was performed between the docked structure and the crystal structure of TCR-Ob.1A12/MBP85–99/HLA-DR2 (10). Docked structure of TCR-Ob.1A12 in cyan, MBP85–99 in brown, and crystal structures of both TCR-Ob.1A12 and HLA-DR2 in blue. The two TCR structures were superimposed by means of structures of MBP85–99.