Abstract
Objective
To determine the relationship between deprivation and hospital admission rates for unintentional poisoning, by poisoning agent in children aged 0–4 years.
Design
Cross sectional study of routinely collected hospital admissions data.
Setting
East Midlands, UK.
Participants
1469 admissions due to unintentional poisoning over two years.
Main outcome measure
Hospital admission rates for unintentional poisoning. Incidence rate ratios (IRRs) comparing hospital admission rates for poisoning in the most and least deprived electoral wards.
Results
Children in the most deprived wards had admission rates for medicinal poisoning that were 2–3 times higher than those in the least deprived wards (IRR 2.49, 95% CI 1.87 to 3.30). Admission rates for non‐medicinal poisoning were about twice as high in the most compared to the least deprived wards (IRR 1.77, 95% CI 1.19 to 2.64). Deprivation gradients were particularly steep for benzodiazepines (IRR 5.63, 95% CI 1.72 to 18.40), antidepressants (IRR 4.58, 95% CI 1.80 to 11.66), cough and cold remedies (IRR 3.93, 95% CI 1.67 to 9.24), and organic solvents (IRR 3.69, 95% CI 1.83 to 7.44).
Conclusions
There are steep deprivation gradients for admissions to hospital for childhood poisoning, with particularly steep gradients for some psychotropic medicines. Interventions to reduce these inequalities should be directed towards areas of greater deprivation.
Keywords: hospital admission, poisoning, children, socioeconomic gradients
Poisoning is a significant child health problem. In the UK, poisoning incidents resulted in an estimated 24 887 attendances at Accident and Emergency departments1 in children aged 0–4 years in 2002. Of the 19 269 hospital admissions for unintentional poisoning in England in the year April 2002/March 2003, 6658 (35%) occurred among those aged 0–14 years.2 Eighty percent of admissions in this age group involve children under 5 years.3 Deaths from unintentional poisoning are, however, rare.4 Three quarters of admissions of children aged 0–14 years for unintentional poisoning result from ingestion of medicinal products.2 Common poisoning agents resulting in hospital attendance in children aged 0–4 years include analgesics, anxiolytics, cough and cold remedies, oral contraceptives, anti‐infectives, bleach, detergents, disinfectants, petroleum based products, cosmetics, and pesticides.5,6 Although most hospital attendances do not require admission, it may be indicated for ingestion of more toxic substances including pesticides, corrosives, paracetamol (acetaminophen), salicylate, or tricyclic antidepressants.7,8
There are pronounced socioeconomic gradients for childhood poisonings. In the eight years from 1985 to 1992, for children aged 0–15 years, those in families of unskilled workers (social class 5) had a higher risk of death due to poisoning than those from families of non‐manual workers.9 Recent studies from England and Wales found significantly higher hospital admission rates for poisoning in children living in the most deprived areas.3,10 Socioeconomic gradients have also been found for Accident and Emergency department attendances for unintentional childhood injuries.11,12 However, little has been published on socioeconomic gradients for poisoning by specific substances.
If interventions to reduce inequalities in admissions are to be effective, those substances for which inequalities are the greatest need to be identified. We have therefore undertaken this study to examine the relation between deprivation and specific poisoning agents among children aged 0–4 years.
Method
Study design
Cross sectional study of routinely collected hospital admissions data for unintentional poisoning, 1995–97.
Case selection and pre‐aggregation data manipulation
Our target population consisted of all admissions due to unintentional poisonings from 862 electoral wards in the former National Health Service Trent Region in the East Midlands, UK for all children aged 0–4 years between 1 April 1995 and 31 March 1997. All admissions for poisoning in this region were to National Health Service hospitals providing care free of charge. Hospitals used the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10) coding system throughout this period.13 Our earlier study3 identified admissions due to unintentional injury in children aged 0–4 years between 1992 and 1997. Admissions were identified from this dataset if they had an ICD10 external cause code indicating unintentional poisoning (X40–X49 inclusive) or a diagnosis code for poisoning (T36–T65 inclusive).
Where an admission involved poisoning by multiple substances, it was included as a single admission in the overall analysis of all poisonings and was also included in the analysis for each substance. Where there was no poisoning diagnosis code, but there was a poisoning external cause code, we mapped this to the corresponding diagnosis code. Data manipulation before aggregation by ward was undertaken using SPSS version 11.14
Aggregation and manipulation of data
We linked each patient, via their postcode of residence, to their electoral ward. Patient level data were aggregated by electoral ward to give numbers of admissions by poisoning agent in each ward. Ward level Townsend scores, derived from four variables from the 1991 Census, were used as a proxy for material deprivation. The Townsend score is a measure of multiple deprivation widely used in the UK.15 Data on other ward level characteristics including distance of ward centroid from the nearest hospital, population, ethnicity, and rurality were obtained as described elsewhere.3
Numbers and rates of admissions were calculated for each category of poisoning agent for each age group. The denominators for rates were ward populations for the relevant age group from the 1991 Census.
Ward Townsend scores were divided into fifths for analyses of all agents, all medicinal and all non‐medicinal agents, and into thirds for other analyses due to smaller numbers.
Data analysis
We used negative binomial regression to determine univariate and multivariable incidence rate ratios (IRRs) with 95% confidence intervals for admission rates for all poisonings and selected agents by fifths or thirds of Townsend score. The use of a Poisson model was rejected because of overdispersion in the data. Tests for trend were undertaken by entering the ward Townsend score into the model as a continuous covariate.
The multivariable analyses were adjusted for distance from the nearest hospital and the percentage of males in the relevant age group in the ward population.5 Models were also adjusted for rurality,16,17 and the percentage of the ward population who were Asian and black18 where these factors were significantly associated with both admission rates and Townsend scores.
Continuous covariates were checked to ensure the linearity of their relationships with admission rates. Regression models were checked for their sensitivity to the removal of outliers and points with high leverage or high influence. A significance level of 5% was used for all analyses. The data manipulation and statistical analysis following aggregation by ward were undertaken using STATA v8.19
Ethical approval
Ethical approval was obtained from Trent Multicentre Research Ethics Committee and all the Local Research Ethics Committees in Trent.
Results
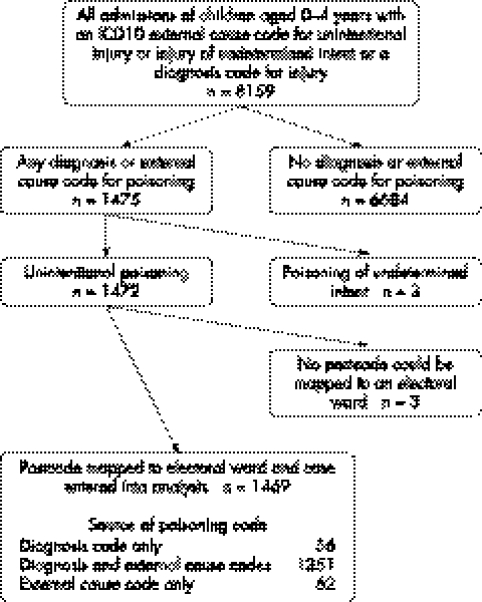
Data were obtained for 1469 admissions for poisoning in children aged 0–4 years. Figure 1 details the selection process. Boys accounted for 57% (837/1469) of the admissions. Numbers and rates of admissions by agent are given in table 1.
Figure 1 Selection of cases.
Table 1 Numbers and rates of admissions for poisoning by various poisoning agents in children 0–4 years old.
| ICD10 diagnosis code and label | Admissions | %* | Rate per 10 000 children per year | Most frequent subcategories of agent within three‐character ICD code (in descending order) |
|---|---|---|---|---|
| T36–T65: All poisonings | 1469 | 100 | 24.35 | |
| T36–T50: All medicinal poisonings | 1024 | 69.7 | 16.98 | |
| T51–T65: All non‐medicinal poisonings | 450 | 30.6 | 7.46 | |
| T39: Non‐opioid analgesics, antipyretics, and antirheumatics | 283 | 19.3 | 4.69 | Paracetamol (acetaminophen); other non‐steroidal anti‐inflammatory drugs; salicylates |
| T42: Anti‐epileptic, sedative‐hypnotic, and antiparkinsonism drugs | 101 | 6.9 | 1.67 | Benzodiazepines; carbamazepine; other antiepileptic and sedative‐hypnotic drugs |
| T42.4 Benzodiazepines | 57 | 3.9 | 0.94 | |
| T43: Psychotropic drugs (not elsewhere classified) | 136 | 9.3 | 2.25 | Non‐monoamine oxidase inhibitor antidepressants; antipsychotics and neuroleptics |
| T43.0–T43.2 Antidepressants | 97 | 6.6 | 1.61 | |
| T45: Systemic and haematological agents (not elsewhere classified) | 104 | 7.1 | 1.72 | Antiallergic and antiemetic drugs; iron; vitamins |
| T48: Agents primarily acting on smooth and skeletal muscles and respiratory system | 115 | 7.8 | 1.91 | Cough medicine; anti‐asthmatics; anti‐common cold drugs |
| T48.3–T48.5 Cough and cold remedies | 74 | 5.0 | 1.23 | |
| T52: Organic solvents | 138 | 9.4 | 2.29 | Other organic solvents; petroleum products |
| T61–T62: Noxious substances eaten as food/seafood | 80 | 5.4 | 1.33 | Parts of plants other than berries or mushrooms; berries; mushrooms |
*Total % is more than 100% because of poisonings by multiple agents.
Results from unadjusted and adjusted negative binomial regressions of admission rates by specific agents on Townsend score are reported in table 2. Significant gradients were found for admission rates for all poisonings, medicinal and non‐medicinal poisonings, with rate ratios increasing with each increase in the level of deprivation (table 2).
Table 2 Incidence rate ratios comparing hospital admission rates by Townsend score for unintentional poisoning by agent in children aged under 5 years.
| ICD10 diagnosis code and label and Townsend bands | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| Incidence rate ratio | (95% CI) | p (test for trend) | Incidence rate ratio | (95% CI) | p (test for trend) | |
| T36–T65: All poisonings | <0.001 | <0.001 | ||||
| Least deprived fifth (−7.66 to −2.95) | 1.00 | 1.00 | ||||
| 2nd (−2.94 to −1.45) | 1.35 | (1.01 to 1.79) | 1.37 | (1.03 to 1.82) | ||
| 3rd (−1.45 to 0.08) | 1.47 | (1.12 to 1.93) | 1.50 | (1.14 to 1.97) | ||
| 4th (0.09 to 2.73) | 1.85 | (1.43 to 2.39) | 1.82 | (1.41 to 2.36) | ||
| Most deprived fifth (2.74 to 11.72) | 2.27 | (1.78 to 2.89) | 2.28 | (1.78 to 2.91) | ||
| T36–T50:All medicinal poisonings | <0.001 | <0.001 | ||||
| Least deprived fifth | 1.00 | 1.00 | ||||
| 2nd | 1.32 | (0.94 to 1.85) | 1.33 | (0.95 to 1.87) | ||
| 3rd | 1.44 | (1.04 to 1.98) | 1.46 | (1.06 to 2.02) | ||
| 4th | 1.99 | (1.48 to 2.67) | 1.96 | (1.45 to 2.64) | ||
| Most deprived fifth | 2.46 | (1.86 to 3.26) | 2.49 | (1.87 to 3.30) | ||
| T51–T65: All non‐medicinal poisonings | 0.008 | 0.01 | ||||
| Least deprived fifth | 1.00 | 1.00 | ||||
| 2nd | 1.39 | (0.87 to 2.20) | 1.41 | (0.89 to 2.25) | ||
| 3rd | 1.46 | (0.94 to 2.28) | 1.50 | (0.96 to 2.34) | ||
| 4th | 1.53 | (1.00 to 2.33) | 1.48 | (0.97 to 2.26) | ||
| Most deprived fifth | 1.77 | (1.19 to 2.64) | 1.77 | (1.19 to 2.64) | ||
| T39: Non‐opioid analgesics, antipyretics and antirheumatics† | 0.07 | <0.001 | ||||
| Least deprived third (−7.66 to −1.93) | 1.00 | 1.00 | ||||
| 2nd (−1.93 to 0.81) | 1.17 | (0.77 to 1.78) | 1.18 | (0.79 to 1.78) | ||
| Most deprived third (0.82 to 11.72) | 1.54 | (1.06 to 2.25) | 1.92 | (1.31 to 2.81) | ||
| T42.4: Benzodiazepines | <0.001 | 0.001 | ||||
| Least deprived third | 1.00 | 1.00 | ||||
| 2nd | 1.44 | (0.36 to 5.80) | 1.50 | (0.37 to 6.02) | ||
| Most deprived third | 5.88 | (1.82 to 18.99) | 5.63 | (1.72 to 18.40) | ||
| T43.0–T43.2: Antidepressants | <0.001 | <0.001 | ||||
| Least deprived third | 1.00 | 1.00 | ||||
| 2nd | 3.99 | (1.51 to 10.51) | 3.88 | (1.47 to 10.23) | ||
| Most deprived third | 4.73 | (1.87 to 11.95) | 4.58 | (1.80 to 11.66) | ||
| T45: Systemic and haematological agents (not elsewhere classified)‡ | 0.10 | 0.38 | ||||
| Least deprived third | 1.00 | 1.00 | ||||
| 2nd | 1.07 | (0.55 to 2.05) | 1.05 | (0.53 to 2.07) | ||
| Most deprived third | 1.46 | (0.82 to 2.57) | 1.20 | (0.64 to 2.26) | ||
| T48.3–T48.5: Cough and cold remedies | <0.001 | <0.001 | ||||
| Least deprived third | 1.00 | 1.00 | ||||
| 2nd | 0.96 | (0.33 to 2.79) | 0.95 | (0.33 to 2.76) | ||
| Most deprived third | 3.72 | (1.60 to 8.66) | 3.93 | (1.67 to 9.24) | ||
| T52: Organic solvents‡ | <0.001 | <0.001 | ||||
| Least deprived third | 1.00 | 1.00 | ||||
| 2nd | 1.62 | (0.78 to 3.38) | 1.86 | (0.87 to 3.96) | ||
| Most deprived third | 3.48 | (1.83 to 6.61) | 3.69 | (1.83 to 7.44) | ||
| T61–T62: Noxious substances eaten as food/seafood‡ | 0.40 | 0.49 | ||||
| Least deprived third | 1.00 | 1.00 | ||||
| 2nd | 1.21 | (0.62 to 2.37) | 1.56 | (0.80 to 3.06) | ||
| Most deprived third | 0.98 | (0.52 to 1.83) | 1.81 | (0.89 to 3.67) | ||
*All adjusted results have been adjusted for distance to nearest hospital and percentage of 0–4 year olds who were male.
†Also adjusted for % black and % Asian.
‡Also adjusted for rurality.
For specific agents, hospital admission rates were more than three times higher among the most deprived third of wards than the least deprived wards for benzodiazepines, antidepressants, cough/cold remedies, and organic solvents. The dose‐response relation demonstrated for all poisonings, medicinal and non‐medicinal poisonings was less apparent for poisoning by specific agents. No significant deprivation gradients were found for systemic and haematological agents, and noxious substances eaten as food/seafood.
Discussion
Principal findings
Admission rates for children aged 0–4 years in the most deprived wards were over three times higher than those for children in the least deprived wards for poisoning by benzodiazepines, antidepressants, cough and cold remedies, and organic solvents. Significant deprivation gradients were also found for all medicinal and all non‐medicinal poisonings, and for poisoning by analgesics, antipyretics, and antirheumatics.
Strengths and weaknesses of the study
This is the largest UK study that has examined hospital admissions by specific poisoning agents. The analysis included all available diagnostic fields in the hospital record, allowing analysis of cases that did not include an external cause code (56, 3.8%) and those involving more than one poisoning agent. Although it is possible that some cases without an external cause code may have been intentional rather than unintentional (because this attribute is coded on the external cause code), the number of such cases was small and this is unlikely to be a substantial factor for the 0–4 year old group. The mapping to electoral wards was unsuccessful in only three cases (0.2%). There is no reason to suppose that quality of recording of admissions differed by the deprivation of the area served by the hospital. An area based deprivation measure was used because of the absence of a measure of the deprivation of individual patients. Caution should therefore be used in making inferences concerning the deprivation of individual patients.
It is possible that factors such as bed availability, local guidelines, the availability of observation or short stay beds, or social factors may have influenced doctors' decisions to admit poisoned children.20 Such factors would be expected to play a greater role in the decision to admit for poisoning by potentially less toxic agents than for that by potentially more toxic agents. Ability to pay for treatment was not a factor because in the UK all admissions for childhood poisoning are to publicly funded hospitals providing care free of charge. However we found particularly steep socioeconomic gradients for poisoning by substances that are potentially very toxic such as antidepressants, so it is unlikely that factors other than the potential toxicity of the ingested substances can completely explain our findings.
Comparison with previous work
We have been unable to find any published work undertaken in the UK on poisoning by specific agents with which to compare our findings. Lyons and colleagues found children aged 0–14 years in the most deprived fifth of wards had a hospital admission rate for poisoning by all agents 1.9 times higher than those in the least deprived fifth of wards, which is consistent with our findings.10
Why do deprivation gradients differ by agent?
The most likely explanation for the deprivation gradients we have found is that there are real differences in exposure to agents between geographical areas. Exposure will be a function both of the quantity of toxic substances kept in homes and the ease with which children can access them. Children are exposed to their own medications and those of other family members,21 and socioeconomic gradients in physical and mental health are well documented.22 Hence, in areas where morbidity is high, a greater range and a larger quantity of medication is likely to be found, at least in countries where prescription medicines are state subsidized. Greater exposure in more deprived areas may also result from easier access to medications resulting from overcrowding, inadequate storage facilities, unsafe storage,23 less support with child care, and less use of out of home day care for children.21
Psychotropic medication is a particular concern as not only are there steep social gradients in maternal depression24,25,26 but there is also some evidence to suggest that maternal safety behaviors may be impaired by mental health problems.27 Children living in deprived areas may therefore be exposed to greater quantities of, and have greater access to, psychotropic medication than those in less deprived areas. These factors may explain the steep deprivation gradients for benzodiazepines and antidepressants. Gradients were less steep for non‐opioid analgesics (including paracetamol (acetaminophen) and aspirin) that are likely to be stored in most households regardless of income.
Gradients in admissions for poisoning by non‐medicinal substances are less likely than those for medicines to be attributable to the availability of these products in the home (as many will be ubiquitous) or to higher quantities of such products being kept in homes in more deprived areas. This may explain the finding that gradients for all non‐medicinal poisoning admissions appeared to be less steep than those for medicinal poisonings. However the steep gradient for organic solvents in young children should be investigated further.
Key points
Children in the most deprived electoral wards had admission rates for medicinal poisoning that were 2–3 times higher than those of the least deprived wards.
Admission rates for non‐medicinal poisoning were about twice as high in the most deprived wards compared with the least deprived wards.
Deprivation gradients were particularly steep for benzodiazepines, antidepressants, cough and cold remedies, and organic solvents.
Implications for practice and further research
The deprivation gradients we found suggest that interventions to reduce childhood poisoning should be targeted on deprived areas. Evidence suggests that home safety education can enhance the safe storage of medicines and cleaning products, especially if combined with the provision and fitting of cupboard locks or catches.28 Such education can be undertaken as part of the child health promotion program in primary care. Exposure to medicines may also be reduced by limiting the quantities of medicines supplied and encouraging the safe disposal of unwanted drugs. Pharmacists may also have a role in giving advice about safe storage, selling lockable medicine cabinets and fridge or cupboard locks, advising that child resistant containers are not “child proof”, and on the action to take in the event of a poisoning incident.
The existence of steep deprivation gradients for antidepressants and benzodiazepines is concerning. Tricyclic antidepressants are among the substances that are potentially lethal to young children in 1–2 dose units.30 Recent guidelines on managing depression in primary and secondary care recommend the use of selective serotonin reuptake inhibitors as first line treatment.31 This may help reduce poisoning by tricyclic antidepressants, but the impact of these guidelines will need to be evaluated. Particular effort should be directed towards the prevention of poisoning by psychotropic drugs associated with poor maternal mental health. Closer liaison between the doctor who prescribes the medication, and other health professionals who have a preventive healthcare role and who provide treatment and support for those with maternal mental health problems may facilitate this.
Further work is needed to explore the relationships between deprivation, the volume of prescribed medication, and the incidence of poisoning.
Acknowledgements
We thank Howard Chapman for help in extracting the data and Liz Webber for her work preparing the datafile.
Abbreviations
NHS - National Health Service
IRR - Incidence rate ratio
ICD10 - International Statistical Classification of Diseases and Related Health Problems, 10th revision
Footnotes
Source of funding: Grant from NHS Executive Trent and Broxtowe and Hucknall Primary Care Trust (NHS Research and Development support funding).
Competing interests: none.
References
- 1.Department of Trade and Industry 24th (Final) Report of the Home and Leisure Accident Surveillance System. London: Department of Trade and Industry, 2003
- 2.Department of Health Hospital Episode Statistics 2002/3. London: Department of Health, 2004
- 3.Hippisley‐Cox J, Groom L, Kendrick D.et al Cross sectional survey of socioeconomic variations in severity and mechanism of childhood injuries in Trent 1992–7. BMJ 20023241132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Office for National Statistics Mortality statistics: cause. Review of the Registrar General on deaths by cause, sex and age, in England and Wales, 2002. London: Office for National Statistics, 2003
- 5.Wiseman H M, Guest K, Murray V S.et al Accidental poisoning in childhood: a multicentre survey. 1. General epidemiology. Hum Toxicol 19876293–301. [DOI] [PubMed] [Google Scholar]
- 6.Rfidah E I, Casey P B, Tracey J A.et al Childhood poisoning in Dublin. Ir Med J 19918487–89. [PubMed] [Google Scholar]
- 7.Sibert J R, Routledge P A. Accidental poisoning in children: can we admit fewer children with safety? Arch Dis Child 199166263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad S, Southall D.Pocket emergency paediatric care. London: BMJ Books, 2003
- 9.Roberts I. Cause specific social class mortality differentials for child injury and poisoning in England and Wales. J Epidemiol Community Health 199751334–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons R A, Jones S J, Deacon T.et al Socioeconomic variation in injury in children and older people: a population based study. Inj Prev 2003933–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alwash R, McCarthy M. Accidents in the home among children under 5: ethnic differences or social disadvantage? BMJ (Clin Res Ed) 19882961450–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing G, Logan S. Patterns of unintentional injury in childhood and their relation to socio‐economic factors. Public Health 1999113291–294. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation International statistical classification of diseases and related health problems: tenth revision. Geneva: World Health Organization, 1992 [PubMed]
- 14.SPSS for Windows [program] 11th version. Chicago: SPSS Inc, 2002
- 15.Hoare J. Comparison of area‐based inequality measures and disease morbidity in England, 1994–1998. Health Stat Q 20031818–24. [Google Scholar]
- 16.Carstairs V, Morris R.Deprivation and health in Scotland. 1st ed. UK: Aberdeen University Press, 1991
- 17.Hjern A, Ringback‐Weitoft G, Andersson R. Socio‐demographic risk factors for home‐type injuries in Swedish infants and toddlers. Acta Paediatr 20019061–68. [DOI] [PubMed] [Google Scholar]
- 18.Trinkoff A, Baker S. Poisoning hospitalizations and deaths from solids and liquids among children and teenagers. Am J Public Health 198676657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.STATA for Windows [program] 8th version. College Station, Texas: STATA Corp, 2003
- 20.Cryer P C, Jarvis S N, Edwards P.et al Why the government was right to change the ‘Our Healthier Nation' accidental injury target. Public Health 2000114232–237. [PubMed] [Google Scholar]
- 21.Petridou E, Polychronopoulou A, Kouri N.et al Unintentional childhood poisoning in Athens: a mirror of consumerism? J Toxicol Clin Toxicol 199735669–675. [DOI] [PubMed] [Google Scholar]
- 22.Acheson D.Independent inquiry into inequalities in health. London: Stationery Office, 1998
- 23.Hapgood R, Kendrick D, Marsh P. How well do socio‐demographic characteristics explain variation in childhood safety practices? J Public Health Med 200022307–311. [DOI] [PubMed] [Google Scholar]
- 24.Baker D, Taylor H. The relationship between condition‐specific morbidity, social support and material deprivation in pregnancy and early motherhood. ALSPAC Survey Team. Avon Longitudinal Study of Pregnancy and Childhood. Soc Sci Med 1997451325–1336. [DOI] [PubMed] [Google Scholar]
- 25.Seguin L, Potvin L, St‐Denis M.et al Depressive symptoms in the late postpartum among low socioeconomic status women. Birth 199926157–163. [DOI] [PubMed] [Google Scholar]
- 26.Heneghan A M, Silver E J, Bauman L J.et al Depressive symptoms in inner‐city mothers of young children: who is at risk? Pediatrics 19981021394–1400. [DOI] [PubMed] [Google Scholar]
- 27.Leiferman J. The effect of maternal depressive symptomatology on maternal behaviors associated with child health. Health Educ Behav 200229596–607. [DOI] [PubMed] [Google Scholar]
- 28.Clamp M, Kendrick D. A randomised controlled trial of general practitioner safety advice for families with children under 5 years. BMJ 19983161576–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson M, Kendrick D, Coupland C.et al Providing child safety equipment to prevent injuries: randomised controlled trial. BMJ 2005330178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bar‐Oz B, Levicheck Z, Koren G. Medications that can be fatal for a toddler with one tablet or teaspoon. Pediatric Drugs 20046123–126. [DOI] [PubMed] [Google Scholar]
- 31.National Collaborating Centre for Mental Health Depression: management of depression in primary and secondary care. National Institute for Clinical Excellence 2004