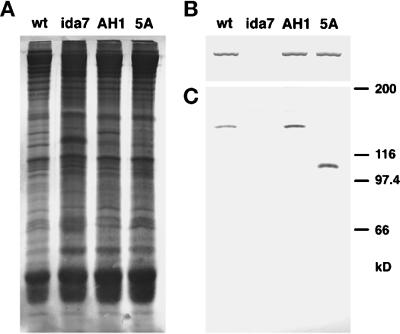
Figure 7.
Assembly of I1 complex polypeptides in wild-type and mutant axonemes. Whole axonemes were isolated from wild-type, the ida7 mutant, and two of the rescued transformants, strain AH1 and strain 5A, and then loaded in duplicate onto a 7% polyacrylamide gel (25 μg/lane), and blotted to two membranes. (A) The first blot was stained with colloidal gold to confirm that equal amounts of total protein were loaded in all the lanes. (B) The high molecular weight region of the second blot was incubated with an antibody specific for the 1α DHC of the I1 complex. A single, high molecular weight band was seen in the wild-type, AH1, and 5A lanes, but not in the ida7 lane. (C) The bottom of the blot was incubated with an antibody specific for the C-terminal half of the IC140 (Yang and Sale, 1998). No band was detected in the ida7 lane, whereas both wild-type and AH1 contained a single immunoreactive band at approximately 140 kDa. Strain 5A, which was recovered after transformation with a fragment of the IC140 gene, has an immunoreactive band at ∼108 kDa.