Abstract
Background
The diagnosis of spinal tuberculosis (ST) is difficult and it commonly presents at an advanced stage. The management and follow up is complicated by a lack of guidance on the appropriate use and interpretation of spinal magnetic resonance studies (MR).
Aims
A retrospective study was performed at a UK centre to identify the demographic and presenting features of a spinal TB population, their response to treatment, and the value of follow up MR studies.
Patients and Results
Twenty one patients were identified with mean symptom duration of 11 (1.5–36) months having been assessed by a health practitioner on 3.2 (0–10) occasions before referral for investigation for ST. Twenty were born outside the UK. Their mean duration of residence in the UK was 6.67 (0.75–20) years, and six (32%) were resident for more than 10 years. Most (85.7%) did not have a medical history and one was HIV positive. Back pain, neurological, and constitutional symptoms were found in 100%, 29%, and 38% respectively. Musculoskeletal and neurological signs were found in 29% and 19% respectively. Spinal MR performed between 6 and 12 months suggests that six months of chemotherapy (for a fully sensitive organism) may not be sufficient to achieve disease resolution.
Conclusions
Awareness of the demographic, clinical, and laboratory features of an ST population may facilitate earlier diagnosis. Guidance is required on the appropriate use and interpretation of MRI in the follow up of these patients.
Keywords: spinal tuberculosis, diagnosis, duration, management
Skeletal disease is the second most common extra‐pulmonary manifestation of tuberculosis (TB), affecting 3%–5% of patients, half of whom have spinal column involvement1 and its prevalence is increasing in developed countries.2 This change is attributable to the contribution of Black African3 and Indian4 subcontinent immigrants to recipient populations and an increase in the prevalence of HIV infection.5
Spinal TB results in significant potential morbidity and mortality6 and delay in diagnosis is associated with increased frequency and severity of complications.6
The latter occurs because of the vague nature of the presenting symptoms and paucity of neurological symptoms and signs7,8 (especially in the early stages of disease). There is also a lack of awareness of the disease because of a low overall total population prevalence of TB9 consequent to the heterogeneous spread of affected ethnic minority groups,3,4 predominantly in urban areas.
International guidelines advocate the treatment of uncomplicated (HIV negative/fully sensitive M tuberculosis) spinal tuberculosis for a period of six months with multiple drug regimens based on rifampicin and isoniazid.10,11 This is based on the multicentre randomised controlled trials of the Medical Research Council (UK).12,13,14 However, compliance with these guidelines in terms of the duration of treatment seems to be poor internationally.15
Furthermore, there is little guidance on the appropriate use and interpretation of MR spine examination in the follow up of these patients, given that plain film radiography was used during the MRC trials and not MR studies.12,13,14 The aims of our study were to examine the presenting features of a population of patients suffering from spinal TB from a large urban United Kingdom teaching hospital, their subsequent management, and nature of follow up investigations.
Patients and methods
Cases of spinal TB were identified retrospectively by examining the records of the TB clinic of Guy's and St Thomas' Hospitals from 1 January 1999 until 30 March 2004. Demographic data, including ethnicity, and duration of residence in the UK and the details of their presenting complaints and respective histories were recorded. The duration of symptoms and number of assessments by health practitioners (general practitioners, accident and emergency department doctors, and hospital consultants) before the consideration of a diagnosis of TB was noted for each patient from the medical history reported in the patient records.
Information regarding the method of diagnosis, initial therapy and subsequent changes, the results of Ziehl‐Nielson stain and TB culture and organism sensitivities as well as baseline and subsequent follow up laboratory, MR investigations both from the patients' medical records and the hospital computerised laboratory records system were noted.
Changes on follow up MR spine examinations were correlated with clinical findings.
The number of patients who completed treatment was noted and outcomes recorded for each patient.
Results
Demographic and clinical and laboratory characteristics
Twenty three subjects with spinal TB were identified from a total of 709 cases (3.24%) with TB from the study period of 64 months. Sufficient data were available on 21, 13 of whom were male and of mean age 35.1 (18–55) years (table 1). Two were white (English and Portuguese) and 19 were born outside the UK. For 19 patients on whom domiciliary data were available, the mean time of residence in the UK was 6.67 (0.75 to 20) years and six were resident for more than 10 years (table 1). Serological examination for HIV was positive in 1 of 10 patients tested, one patient was an injecting drug user (HIV negative), and otherwise there were no risk factors for immune compromise. Only three additional patients had a medical history.
Table 1 Demographic and clinical details of patients presenting with spinal tuberculosis.
| Number | Sex | Age at diagnosis | Country of birth | Duration resident in the UK | Retroviral status | Additional medical history |
|---|---|---|---|---|---|---|
| 1 | F | 35 | Somalia | 7 | Not tested | Nil |
| 2 | M | 49 | Vietnam | 15 | Not tested | Asthma |
| 3 | F | 42 | Nigerian | 15 | Not tested | Hypertension |
| 4 | M | 31 | Portugal | Unknown | Negative | Injecting drug use |
| 5 | M | 52 | Sri Lanka | 4 | Not tested | Nephrolithiasis |
| 6 | F | 28 | UK | Not tested | Nil | |
| 7 | F | 24 | Eritrea | 0.75 | Not tested | Nil |
| 8 | M | 36 | Somalia | 0.25 | Not tested | Nil |
| 9 | M | 34 | Sierra Leone | 11 | Negative | Nil |
| 10 | F | 31 | Ivory Coast | 1 | Positive | Nil |
| 11 | M | 31 | Nigerian | 4 | Nil | |
| 12 | M | 34 | Vietnam | 15 | Negative | Nil |
| 13 | M | 32 | Ivory Coast | 2 | Negative | Nil |
| 14 | F | 55 | West Africa | 20 | Not tested | Nil |
| 15 | M | 38 | Nepal | 2.5 | Negative | Nil |
| 16 | M | 31 | Somalia | 10 | Negative | Nil |
| 17 | F | 18 | Eritrea | 2 | Negative | Nil |
| 18 | F | 30 | Pakistan | Unknown | Not tested | Nil |
| 19 | M | 35 | Sri Lanka | 7 | Not tested | Nil |
| 20 | M | 33 | Zimbabwe | 12 | Negative | Nil |
| 21 | M | 39 | UK | – | Negative | Nil |
Back pain was the commonest clinical presentation (n = 21, 100%), six (29%) presented with neurological symptoms (lower limb paraesthesia in three, upper limb weakness in one, and lower limb weakness in two). Ten patients (48%) complained of one or more constitutional symptom suggestive of tuberculous infection (six (29%) night sweats, five (24%) fever, five (24%) significant weight loss, and one (5%) rigors) (table 2).
Table 2 Presenting symptoms, signs, symptom duration, vertebral column involvement, pathogenic mycobacteria, and antibiotic sensitivities of patients with spinal tuberculosis.
| Number | Presenting symptoms | Presenting signs | Const Symt | Duration of symptoms (months) | Vertebral column involvement | Culture | Sensitivity | ||
|---|---|---|---|---|---|---|---|---|---|
| Msk | Neuro | Msk | Neuro | ||||||
| 1 | Y | Y | 23 | T8–T9 | Neg | – | |||
| 2 | Y | Y | Y | Y | 30 | T2–T3 | MTB | S resistant | |
| 3 | Y | 12 | L2–L3 | MTB | FS | ||||
| 4 | Y | Y | Y | Y | 2 | T5–T8 | MTB | FS | |
| 5 | Y | Y | Y | Y | 6 | T10–T11 | Neg | – | |
| 6 | Y | Y | Y | 1.5 | L4–L5 | Neg | – | ||
| 7 | Y | Y | 3 | T4–T5 | Neg | – | |||
| 8 | Y | Y | 12 | N/A | MTB | S resistant | |||
| 9 | Y | Y | Y | 5 | T5–T6 | MTB | FS | ||
| 10 | Y | T12–L1 | MTB | FS | |||||
| 11 | Y | Y | 3 | T9–T12 | MTB | S resistant | |||
| 12 | Y | Y | 2 | S1 | MTB | FS | |||
| 13 | Y | 6 | T7, L2–L3 | Neg | – | ||||
| 14 | Y | Y | Y | 36 | T10–L1 | MTB | FS | ||
| 15 | Y | 5 | T11–T12, L3–L5 | MTB | FS | ||||
| 16 | Y | Y | Y | 3 | T10 | MTB | FS | ||
| 17 | Y | Y | 7 | T10–T11 | MTB | INH resistant | |||
| 18 | Y | Y | 27 | C1–C4 | MTB | FS | |||
| 19 | Y | Y | 6 | T8 | MTB | FS | |||
| 20 | Y | Y | 6 | C1–C2, T3, L3 | MTB (MDR) | R, INH, Z, ETH, S,Cip, resistant | |||
| 21 | Y | Y | N | Y | Y | 18 | T10–12 | MTB | FS |
Msk, musculoskeletal; neuro, neurological; Y, yes; C, cervical; T, thoracic; L, lumbar; MTB, mycobacterium tuberculosis; MDR, multiple drug resistant; FS, fully sensitive; S, streptomycin; INH, isoniazid; R, rifampicin; Z, pyrazinamide; ETH, ethambutol; Cip, ciprofloxacin; Const Symt, constitutional symptoms; N/A, not available.
A neurological deficit was present in four (19%), three of whom had upper motor neuron lesions involving the lower limbs, two had an associated sensory level, and one patient had isolated radicular pain without a motor deficit.
Seven (33%) had evidence of extra‐spinal disease during the course of their investigations (three (14%) pulmonary, one (5%) pleural, one (5%) pelvic, one (5%) military, and one (5%) had a combination of gastrointestinal, lymphatic, and extra‐spinal skeletal disease), five of whom were symptomatic.
Laboratory parameters, before start of treatment showed that all of the patients were hypo‐albuminaemic, and had an increased serum alkaline phosphatase and CRP. Although the mean platelet count was within the normal range, 57% had a thrombocytosis at baseline. The white cell count was normal in 75% of patients but 87.5% had evidence of an anaemia.
Diagnosis
The mean duration of symptoms before diagnosis was 11 (1.5–36) months in the 20 patients for whom this was recorded (table 2). Seven and three patients had symptom durations of greater than one and two years respectively.
The mean number of health practitioner assessments before consideration of and referral for a diagnosis of spinal TB, of the 19 patients for whom this was recorded, was 3.2 (0–10).
In three (14%) patients the diagnosis was made on presentation while seven (33%) were assessed on five or more occasions before an appropriate referral was made.
Radiologically guided vertebral or para‐vertebral biopsy and aspiration was performed in 15 (71%) patients (CT guided 13 (62%), ultrasound guided in two (10%)) and a surgical procedure was performed in four (19%) (one because of a failed CT guided procedure).
The diagnosis was made from extra‐spinal material specimens in four (sputum in two, pleural fluid in one, and a miscarried fetus in one).
Ziehl‐Nielson stain was positive in 15 of 20 (75%) specimens examined, and culture was positive for M tuberculosis in 16 (76%) and negative in five (24%).
Eleven of the specimens isolated (69%) were fully sensitive to all first line antituberculous antibiotics, three (19%) were resistant to streptomycin, one (6%) was isoniazid resistant, and one was resistant to multiple antibiotics (rifampicin, isoniazid, ethambutol, pyrazinamide, and streptomycin) (table 2).
The results of spinal MR studies were available on 20 patients. Multifocal vertebral column disease was found in three (15%) patients on MR examination (table 2). Vertebral disease (TB spondylitis) without disc disease was found in only one patient. The remainder (95%) suffered from spondylodiscitis showing evidence of both vertebral body and disc disease. The mean number of vertebrae involved was 2.6, but six (30%) patients had disease involving four. The commonest site of disease was the lower thoracic (T10–T12) and the mid‐lumbar vertebrae (L3).
MR evidence of spinal cord compression, of cauda equina compression, and of radicular disease was found in 13 (65%), two (10%), and two (10%) patients respectively. Two had evidence of a myelopathy on MR examination (both spinal cord compression). However, only four had evidence of a suggestive neurological deficit on clinical examination.
Treatment
All patients received standard therapy with rifampicin, isoniazid, ethambutol, and pyrazinamide for two months and subsequently rifampicin and isoniazid unless mycobacterial sensitivities suggested otherwise (table 3).
Table 3 Treatment and outcomes of patients with spinal tuberculosis.
| Number | Treatment | Surgery | Corticosteroids | Duration of treatment | Residual symptoms and signs |
|---|---|---|---|---|---|
| 1 | RIPE | None | 12 | None | |
| 2 | RIPE | T2/T3 vertebrectomy, bone graft, posterior instrumented fusion (C4–T7) | Y | 15 | Back pain |
| 3 | RIPE | None | Y | 12 | |
| 4 | RIPE | T6 vertebrectomy, posterior spinal fusion with pedicular screws and rod instrumentation from T3 to T9 | 14 | UMN signs lower limbs | |
| 5 | RIPE | None | Y | 13 | Kyphosis T8–10 |
| 6 | RIPE | None | 14 | Back stiffness | |
| 7 | RIPE | None | 9 | Back pain | |
| 8 | RIPE | None | 12 | None | |
| 9 | RIPE | None | 9 | L'hermittes phenomenon | |
| 10 | RIPE | None | 12 | None | |
| 11 | RIPE | I and D of paravertebral mass | Y | T/O | |
| 12 | RIPE | None | T/O | ||
| 13 | RIPE | None | Y | T/O | |
| 14 | RIPE | T11 and 12 debridement and bone graft | 9 | None | |
| 15 | RIPE | None | 12 | None | |
| 16 | RIPE | None | Y | 12 | Gibus (T10), scoliosis, limited flexion, reduced sensation S1 dermatome |
| 17 | RPE | None | 20 | Gibbus and mild kyphosis | |
| 18 | RIPE | Transoral decompression of cervical abscess. Removal of odontoid process and lateral edge of the atlas. | 13 | Neck shortening, limited lateral rotation bilateral (10°) and flexion/extension | |
| 19 | RIPE | None | 24 | Back pain | |
| 20 | RIPE then ETH, cycloserine, PAS, clarithro | Rod instrumentation C1–C4 | 34 (T/O) | Dysphagia | |
| 21 | RIPE | None | 18 | Pleuritic chest pain | |
RIPE, rifampicin, isoniazid, pyrazinamide, ethambutol; I and D, incision and drainage; T, thoracic; Y, yes; T/O, treatment ongoing; UMN, upper motor neurone lesion.
Oral corticosteroid therapy was given to six (28.6%) patients for one month (table 3). Two had spinal cord compression alone, two had spinal cord compression and evidence of myelopathy, one had spinal cord compression and a radiculopathy, and one had cauda equina compression.
Surgical intervention was required in six (28.7%) patients in whom five were therapeutic procedures (table 3). Of those who had a therapeutic surgical procedure, four had no neurological symptoms or signs on completion of their antituberculous treatment regimen, two had residual musculoskeletal symptoms and signs.
Of the 17 who have completed therapy, the mean duration of treatment was 13.0 (9 to 24) months (table 4). Seven patients (41%) had persistent musculoskeletal symptoms, two (12%) had neurological symptoms, and one complained of dysphagia (cervical spine TB) on completion of treatment (table 3). The remainder were asymptomatic.
Table 4 Follow up spinal magnetic resonance studies from six months after start of treatment months onwards.
| Number | Six months | Nine months | Twelve + months |
|---|---|---|---|
| 1 | No evidence of infection | ||
| 3 | No evidence of infection | ||
| 6 | Persistent L4/5 discitis | ||
| 7 | No evidence of infection | ||
| 9 | Persistent T11/12 discitis | ||
| 15 | No evidence of infection | ||
| 16 | Persistent vertebral body abscess, T11, 12, L4–5 | ||
| 17 | Persistent vertebral body oedematous change, T10–11 with persistent disc enhancement at T10/11. | ||
| 18 | No evidence of infection | ||
| 20 | Persistent vertebral body abscess, C1–2 and T3 with cord compression and T3 paraspinal abscess | Progression of C1–2 and T3 abscess, worsening cord compression |
Abnormal results indicative of persistent disease activity are shown in bold type.
Follow up MR studies
Follow up MR spine examination was performed in 14 patients (70%). These were performed between one and six months in five, and between six months and one year in 10 patients (table 4, figs 1 and 2). In four of the five patients MR examinations between one and three months showed disease resolution. However, spinal MR performed in one patient at three months because of recent onset lower back pain (which had initially resolved after start of, and full compliance with treatment for a fully sensitive MTB) showed extending osteomyelitis (patient 12) and a large pre‐sacral collection. Surgery showed a sterile abscess with no evidence of active disease on Ziehl‐Nielson stain or subsequent culture.
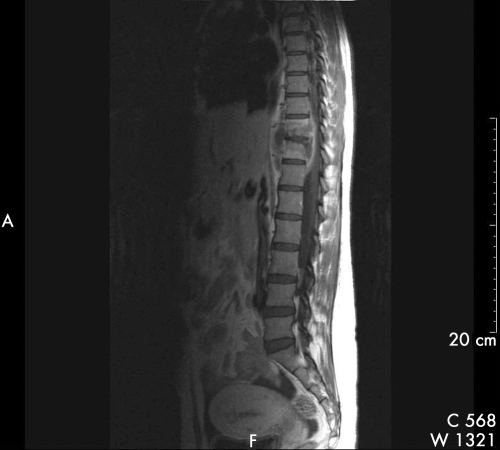
Figure 1 Saggital T1 weighted MR spine with contrast (gadolinium), of patient 17 showing T10/11 intervertebral disc enhancement with disc space narrowing, T10 and T11 vertebral body abscess, paraspinal mass with anterior displacement of the aorta, and extradural component anterior to the spinal cord resulting in compression.
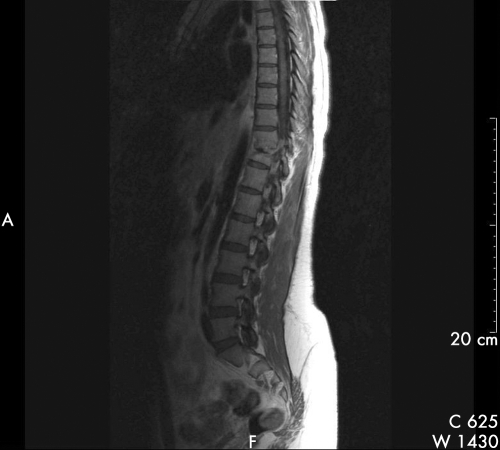
Figure 2 Saggital T 1 weighted MR spine with contrast (gadolinium) of patient 17, after nine months of treatment for spinal tuberculosis, showing persistent enhancement of T10/11 intervertebral disc with persistent oedematous change at T10 and T11 vertebral bodies in addition to spinal canal narrowing. The anterior para‐vertebral and extra‐dural masses have reduced in size.
Of the MR spine examinations performed between 6 and 12 months, 5 of 10 (50%) patients had radiological evidence of persistent disease activity (patients 6, 9, 16, 17, 20). The changes found were suggestive of infective discitis in three (patient 9 at six months, patient 17 at nine months, and patient 13 at 12 months), vertebral body abscess in one (patient 16 at nine months), and vertebra1 body oedema in one (patient 17 at nine months). Multi‐focal intraosseous abscess with persistent cord compression was found in patient 20 at 9 and 12 months.
Patient 20 subsequent to starting treatment was found to have multiple drug resistant M tuberculosis, however the remaining four had a fully sensitive organism and were fully compliant with treatment.
Discussion
The significant contribution of members of ethnic minorities to our spinal TB population is consequent on the large ethnic/immigrant composition of our local population16 and differs from studies from other developed countries.7,8,17,18,19 These groups have a considerably higher prevalence of TB reflecting that of their country of origin in comparison with members of UK born white and non‐white populations.3,4 Furthermore, extra‐pulmonary manifestations of TB are more frequent among non‐white people.1
The fact that 32% of the patients in this series did not develop (were not diagnosed with) spinal TB until after 10 years of residence in the UK suggests that the increased risk of developing TB for members of immigrant communities may not revert to that of the recipient population for longer than the five years previously thought.4,20
The large spread in symptom duration (up to 36 months) before diagnosis reflects the variable and chronic nature of this disease and may also be a function of a delay in self presentation. However, we have also shown a high frequency of health practitioner assessments before consideration of a diagnosis of spinal TB (33% required five or more assessments before the definitive referral for investigation was made). This is a feature that has not changed in the past five decades9 despite advances in diagnostic technology. The difficulty in recognising spinal TB stems from the fact that consultations for chronic back pain represent a large proportion of primary care workload,21 the causes are multiple22 and in up to 80% of cases, a cause cannot be found.23 Furthermore, it is not helped by the fact that, while complaining of chronic back pain, only one third of the patients complained of neurological or constitutional symptoms and less than a quarter had neurological signs. In fact 61% of the patients in this series had no organ specific symptoms or signs (spinal or extra‐spinal) apart from back pain.
However, it is striking that the population we have described who suffer from spinal TB is comparatively young (mean age 35 years), predominantly healthy, and without risk factors for immune compromise, non‐white (90%), and almost all born outside the UK (predominantly in countries where TB is endemic). Notably of the two white patients in this series, one was an injecting drug user while the second shared a room with an index case of pulmonary TB for a year.
Only 1 of the 10 patients tested for HIV serology was positive in our study. We acknowledge that the threshold for advice for HIV testing has diminished since the beginning of our study,24 but among the remaining patients who were not tested, there was no social, clinical, or laboratory indication to advise such a test. Of the few with a medical history, it was not sufficiently significant to have been responsible for their development of spinal TB.
We therefore suggest that spinal TB should always be considered a possibility in the differential diagnosis of chronic back pain with or without constitutional, neurological, or musculoskeletal symptoms and signs on examination, in young, otherwise healthy patients from ethnic minorities, particularly those who originate from countries with high endemic rates of TB.
Duration of residence in the adoptive country of greater than five years seems to render them no less likely of presenting with spinal TB.
The diagnosis of spinal TB in this population is even more important when baseline laboratory examination shows hypoalbuminaemia, a raised serum C reactive protein, and alkaline phosphatase and/or anaemia.
MR was the radiological investigation of choice for all patients because of its capacity to precisely delineate the anatomical location of disease in multiple planes.25,26
MR studies showed a far higher incidence of spinal cord and cauda equina compression than did clinical examination in this case series. Although none of the patients in this series suffered from worse than Frankel D27 spinal cord pathology (sparing of sensation and non‐useful motor function distal to the spinal lesion), a decision to intervene surgically was made on its basis in 30% of cases. Furthermore the detection of sub‐clinical spinal cord compression by MR permitted the targeted prescription of corticosteroids in five patients who were not felt to necessitate a therapeutic surgical procedure.
MR studies also showed additional unknown extra‐spinal disease.
Radiologically guided percutaneous biopsy, usually by CT was the predominant method of obtaining a microbiological diagnosis. In only 10% of cases was a surgical procedure used, reflecting differences between the MRC trials and current UK practice.12
The most frequent site of involvement was T10–12 and L3, although all vertebra from C1 to S1 were involved. However, only 5% of cases had vertebral disease without disk involvement (spondylitis). Most patients presented with spondylodiscitis contrasting with the findings of Pertuiset8 who found spondylitis without disc involvement to be more frequent among foreign born than endogenous (French) patients.
A positive Ziehl‐Neilson stain and culture rate of 75% and 76% respectively, is comparable to that previously noted in studies using radiologically guided percutaneous biopsy.8,17,18,28,29
However, studies of the MRC performed in Zimbabwe,30 Hong Kong,31 and South Africa32 had a higher proportion of positive mycobacterial cultures.
This may reflect the number and volume of specimens taken for laboratory analysis,33 given that a surgical rather than a percutaneous radiological approach was used to obtain diagnostic material.
The prevalence of single drug resistance was higher at 25% while that of isolated isoniazid resistance (6.25%) was similar to the previous national prevalence in England and Wales.34 The presence of isolated streptomycin resistance at 21% was unusually high, but may reflect the known higher level of single drug resistance noted in London compared with the rest of England and Wales.34 The presence of MDR‐TB in one patient resulted in a delayed improvement (because of inadequate treatment) and a prolonged and complicated course of treatment.
This underscores the importance of determining the identity and sensitivity of the mycobacteria, not just from a public heath and epidemiological viewpoint but also from a treatment management viewpoint.
There is no guidance on the appropriate imaging modality and frequency of use in the follow up of spinal TB.10,11 Follow up in the MRC trials is based on plain film radiology of the spine.12,13,14 In 50% of those who had spinal MR examinations performed at or after six months there was still evidence of persistent disease activity, necessitating prolongation of treatment (table 4, fig 2).
Although one of these five had MDR‐TB of the spine, the remaining four had a fully sensitive organism, reported to be fully concordant with chemotherapy and had clinical and laboratory evidence of disease resolution.
Although there MRC trials have shown persistent changes in plain radiological appearances of the vertebral column up to three years after completion of treatment, these changes were attributed to new bone formation and ankylosis.35 Self limiting abscesses have been described in the MRC series affecting up to 8% of patients. These however resolved within six months of chemotherapy.13 There is currently no guidance on soft tissue or vertebral changes noted on MR spine examination, during the course of, or after antituberculous therapy.
Both the British and American Thoracic Societies, based on the evidence of the MRC trials, advocate short course chemotherapy (six months) for adult uncomplicated fully sensitive spinal TB.10,11
However, the evidence presented in this case series suggests that in a significant proportion of patients, longer durations of treatment may be required as their MR scans were abnormal even though there is apparent disease resolution clinically. Furthermore, time course studies of the MR changes of spinal TB need to be performed to achieve consensus on the interpretation and use of this powerful modality in the follow up of spinal TB patients.
The opportunity to diagnose spinal TB early is often missed by health practitioners. Clinical suspicion should be aroused by the complaint of chronic back pain even in the absence of neurological symptoms and signs, in young adults from immigrant ethnic minorities irrespective of their duration of domicile in their adoptive country. A history of one or more of the systemic symptoms of night sweats, fever, and weight loss will be elicited in less than half, but all should have laboratory tests for an inflammatory disease process.
Further guidance is required on the appropriate use and interpretation of MR studies in the follow up of spinal TB and the duration of treatment, in the light of abnormal MR findings.
Abbreviations
TB - tuberculosis
MR - magnetic resonance
Footnotes
Funding: none.
Conflicts of interest: none.
References
- 1.Medical Research Council Medical Research Council National Survey of Tuberculosis Notifications in England and Wales in 1983: characteristics of disease. Tubercle 19886819–32. [DOI] [PubMed] [Google Scholar]
- 2.Rezai A, Lee M, Cooper P.et al Modern management of spinal tuberculosis. Neurosurgery 19953687–89. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D, Watson J, Charlett A.et al Tuberculosis in England and Wales in 1993: results of a national survey. Thorax 1997521060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ormerod L, Charlett A, Gilham C.et al Geographical distribution of tuberculosis in national surveys of England and Wales in 1988 and 1993: report of the Public Health Laboratory Service/British Thoracic Society/Department of Health Collaborative Group. Thorax 199853176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellis J. Human immunodeficiency virus and osteoarticular tuberculosis. Clin Orthop 200239827–31. [DOI] [PubMed] [Google Scholar]
- 6.Jain A. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop 200239875–84. [DOI] [PubMed] [Google Scholar]
- 7.Fam A, Rubenstein J. Another look at spinal tuberculosis. J Rheumatol 1993201731–1740. [PubMed] [Google Scholar]
- 8.Pertuiset E, Beaudreuil J, Liote F.et al Spinal tuberculosis in adults. A study of 103 cases in a developed country, 1980–1994. Medicine 199978309–320. [DOI] [PubMed] [Google Scholar]
- 9.Walker G. Failure of early recognition of skeletal tuberculosis. BMJ 1968i682–683. [DOI] [PMC free article] [PubMed]
- 10.Joint Tuberculosis Committee of The British Thoracic Society Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax 198853536–548. [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society Centers for Disease Control and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med 2003167603–662. [DOI] [PubMed] [Google Scholar]
- 12.Twelfth report of the Medical Research Council Working Party on Tuberculosis of the spine Controlled trial of short‐course regimens of chemotherapy in the ambulatory treatment of spinal tuberculosis. J Bone Joint Surg (Br) 199375‐B240–248. [DOI] [PubMed] [Google Scholar]
- 13.Tenth report of the Medical Research Council Working Party on Tuberculosis of the Spine A controlled trial of six‐month and nine‐month regimens of chemotherapy in patients undergoing radical surgery for tuberculosis of the spine in Hong Kong. Tubercle 198667243–259. [DOI] [PubMed] [Google Scholar]
- 14.Medical Research Council Working Party on Tuberculosis of the Spine Five‐year assessments of controlled trials of short course chemotherapy regimens of 6, 9 or 18 months' duration for spinal tuberculosis ambulatory from the start or undergoing radical surgery. Int Orthop 19992373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Loenhout‐Rooyackers J, Verbeek A, Jutte P. Chemotheraputic treatment for spinal tuberculosis. Int J Tuberc Lung Dis 20026259–265. [PubMed] [Google Scholar]
- 16.Lambeth Council Census 2001 topic report. http://www.lambeth.gov.uk/statistics
- 17.Janssens J, De Haller R. Spinal tuberculosis in a developed country. Areview of 26 cases with special emphasis on abscesses and neurological complications. Clin Orthop 199025767–75. [PubMed] [Google Scholar]
- 18.Leibert E, Schluger N, Bonk S.et al Spinal tuberculosis in patients with human immunodeficiency virus infection: clinical presentation, therapy and outcome. Tuber Lung Dis 199677329–334. [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum E, Rockswold G, Bergman T.et al Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg 199583243–247. [DOI] [PubMed] [Google Scholar]
- 20.Report of the British Thoracic and Tuberculosis Association Tuberculosis among immigrants related to length of residence in England and Wales. BMJ 1975iii698–699. [DOI] [PMC free article] [PubMed]
- 21.Mc Cormick A, Fleming A, Charlton D.Morbidity statistics from general practice. Fourth national study 1991–1992. London: HMSO, 1995
- 22.Jenner J, Barry M. Low back pain. In: Snaith M, ed. ABC of rheumatology. London: BMJ Books, 199610–13.
- 23.Waddell G. A new clinical model for the treatment of low bak pain. Spine 198712632–644. [DOI] [PubMed] [Google Scholar]
- 24.Bowen E, Rice P, Cooke N.et al HIV seroprevalence by anonymous testing in patients with Mycobacterium tuberculosis and in tuberculosis contacts. Lancet 20003561488–1489. [DOI] [PubMed] [Google Scholar]
- 25.Bell G, Stearns K, Bonutti P.et al MRI diagnosis of tuberculous vertebral osteomylitis. Spine 199015462–465. [DOI] [PubMed] [Google Scholar]
- 26.Griffith J, Kumta S, Leung P.et al Imaging of musculoskeletal tuberculosis: a new look at an old disease. Clin Orthop 200239832–39. [DOI] [PubMed] [Google Scholar]
- 27.Davis L, Warren S, Reid D.et al Incomplete neural deficits in thoracolumbar and lumbar spine fractures. Reliability of Frankel and Sunnybrook scales. Spine 199318257–263. [PubMed] [Google Scholar]
- 28.Colmenero J, Jiminez‐Mejias M, Sanchez‐Lora F.et al Pyogenic, tubercular, and brucellar vertebral osteomylitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis 199756709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lifeso R, Weaver P, Harder E. Tuberculous spondylitis in adults. J Bone Joint Surg 198591405–1413. [PubMed] [Google Scholar]
- 30.Medical Research Council Working Party on Tuberculosis of the Spine A controlled trial of debridement and ambulatory treatment in the management of tuberculosis of the spine in patients on standard chemotherapy: a study in Bulawayo. J Trop Med Hyg 19747772. [PubMed] [Google Scholar]
- 31.Medical Research Council Working Party on Tuberculosis of the Spine A controlled trial of anterior spinal fusion and debridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in Kong Kong. Br J Surg 197461853. [DOI] [PubMed] [Google Scholar]
- 32.Medical Research Council Working Party on Tuberculosis of the Spine A controlled trial of anterior spinal fusion and debridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy: a study in two centres in South Africa. Tubercle 19785976. [DOI] [PubMed] [Google Scholar]
- 33.Allen B, Mitchison D, Darbyshire J.et al Examination of the operation specimens from patients with spinal tuberculosis for tubercle bacilli. J Clin Pathol 198336662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Communicable Disease Surveillance Centre Antimicrobial resistance in England and Wales 2000. London: Public Health Laboratory Service, 2000
- 35.Thirteenth report of the Medical Research Council Working Party on Tuberculosis of the Spine A 15‐year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong. J Bone Joint Surg 199880‐B456–462. [DOI] [PubMed] [Google Scholar]