Fig. 1. PrPSc accumulation induces ER stress and reduces PrP translocation into the ER.
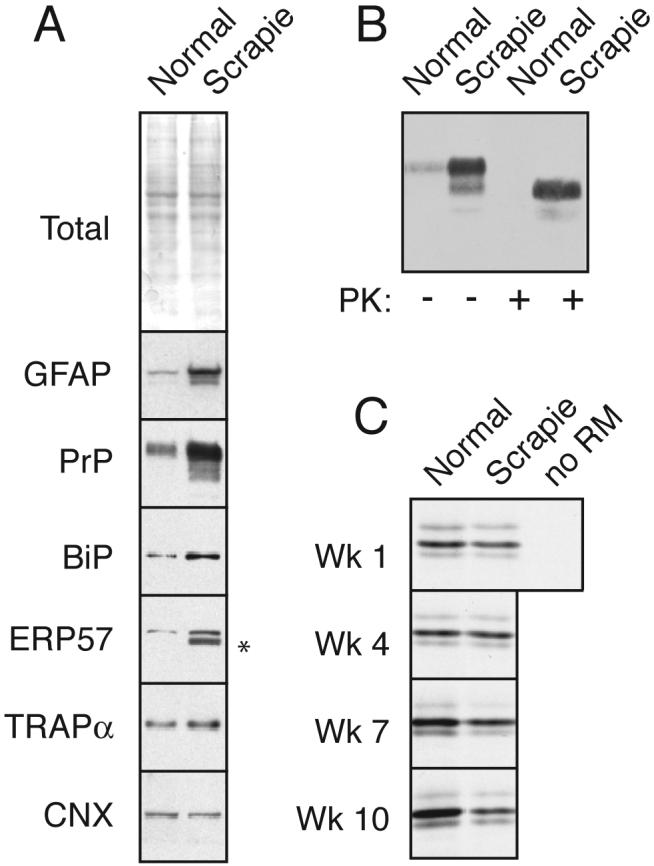
(A) Total brain homogenate from normal and PrPSc-infected hamsters (‘normal’ and ‘scrapie’) were analyzed by staining for total proteins or immunoblotted for the indicated antigens. Asterisk indicates trace IgG heavy chain that occassionally contaminates tissue homogenates from residual blood.
(B) Analysis of PrP from the samples in panel A for resistance to digestion by proteinase K (PK). No protease-resistant PrP was detected in normal tissue even upon gross overexposure of the blot (not shown).
(C) ER microsomes from normal and PrPSc-infected hamsters were used for in vitro translocation assays for PrP. After synthesis with 35S-methionine, the samples were treated with PK to digest non-translocated products, and the protease-protected PrP (indicative of its successful translocation into the ER microsomes) was recovered by immunoprecipitation. Shown are autoradiographs of the translocated PrP. Note that in the absence of membranes (‘-’), full length PrP is not protected. In samples from animals 1 and 4 weeks post-inoculation (before PrPSc accumulation), no difference is observed in normal and scrapie microsomes. By contrast, at a time when PrPSc accumulation is high (7 and 10 weeks; see Sup. Fig. 1), translocation is significantly lower in infected microsomes relative to the uninfected control. Note that each pair of microsomes at every time point were isolated and analyzed in parallel; however, comparisons may not be valid between time points, so the apparent increase in translocation in normal microsomes over time may not be meaningful.
