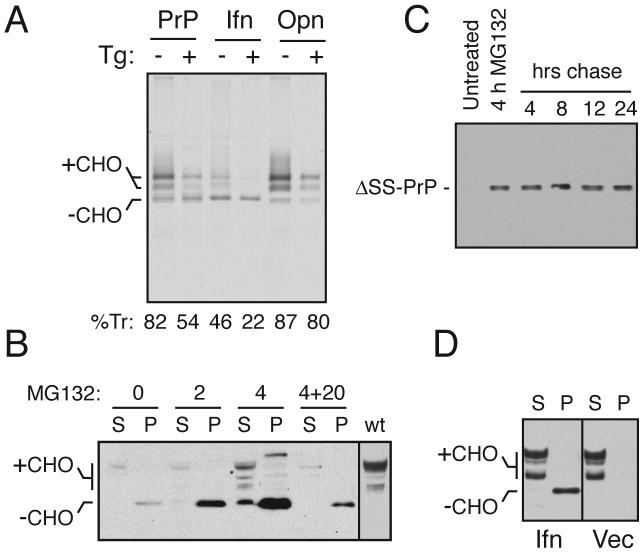
Fig. 4. Ifn-PrP mimics pQC in vivo in the absence of ER stress.
(A) ER translocation of the indicated PrP constructs in transiently transfected Hela cells subjected to acute ER stress (15 min) by Ca+2 depletion using thapsigargin (Tg). Translocation was quantified using relative glycosylation efficiency and is indicated below the respective lanes. The positions of unglycosylated (-CHO) and glycosylated (+CHO) species of PrP are indicated. Note that protein synthesis is reduced in stressed cells due to PERK-mediated phosphorylation of eIF2α.
(B) N2a cells transiently transfected with Ifn-PrP were treated with proteasome inhibitor (10 uM MG132) for 0, 2, or 4 h as indicated and analyzed by immunoblotting. Samples were separated into detergent-soluble (S) and insoluble (P) fractions before analysis. ‘4+20’ indicates samples from cells treated with inhibitor for 4 hours, and cultured in the absence of inhibitor for an additional 20 h. The last lane is a marker for mature PrP from cells expressing wild type PrP.
(C) N2a cells transiently transfected with ΔSS-PrP were treated with proteasome inhibitor (10 uM MG132) for 4 h as indicated, and either harvested immediately, or cultured for an additional 4 to 24 h in the absence of inhibitor. All samples were analyzed for ΔSS-PrP by immunoblotting with 3F4 antibody.
(D) N2a cells transiently transfected with Ifn-PrP or empty vector were separated into detergent-soluble (S) and insoluble (P) fractions before analysis by immunoblot using a PrP antibody that detects both endogenous PrP and Ifn-PrP. Note the lack of changes to endogenous PrP in cells expressing Ifn-PrP (most of which is found in the insoluble fraction as unglycosylated species).