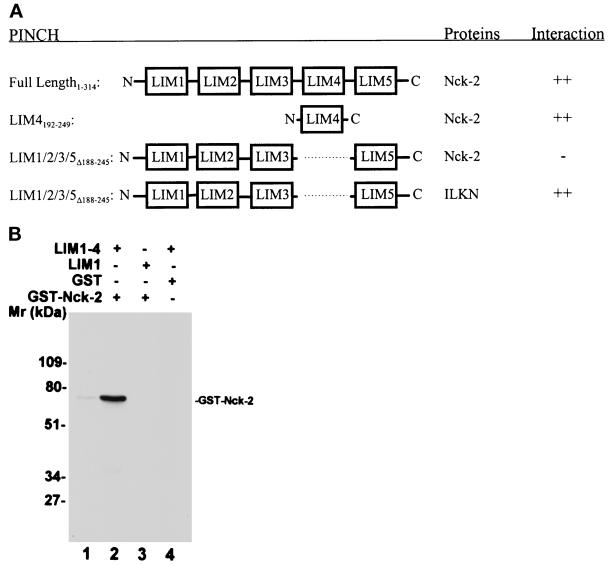
Figure 2.
Analyses of the PINCH-Nck-2 interaction. (A) Yeast two-hybrid binding assays. The cDNAs encoding PINCH sequences were inserted into the pB42AD vector. The cDNAs encoding Nck-2 and ILK sequences were inserted into the pLexA vector. The protein–protein interactions were analyzed by yeast two-hybrid assays as described in MATERIALS AND METHODS. ++, Growth of blue colonies on the leucine-deficient selection medium containing 80 μg/ml X-gal was detected within 1 d; −, no blue colony was detected after 5 d. LIM1/2/3/5▵188–245, PINCH mutant in which the LIM4 domain (residues 188–245) was deleted; LIM4192–249, PINCH mutant containing the LIM4 domain (residues 192–249); ILKN, the N-terminal domain of ILK (residues 1–163). (B) Coprecipitation assays. Affinity-purified GST-Nck-2 (lanes 2 and 3), or GST as a control (lane 4), was mixed with His-tagged PINCH LIM1–4 (residues 1–249)(lanes 2 and 4) or His-tagged PINCH LIM1 (residues 1–70)(lane 3). The GST-Nck-2 was coprecipitated with the His-tagged LIM1–4 and detected by immunoblotting with a polyclonal rabbit anti-GST-Nck-2 antibody as described in MATERIALS AND METHODS. Lane 1 was loaded with 10 ng of affinity- purified GST-Nck-2 fusion protein.