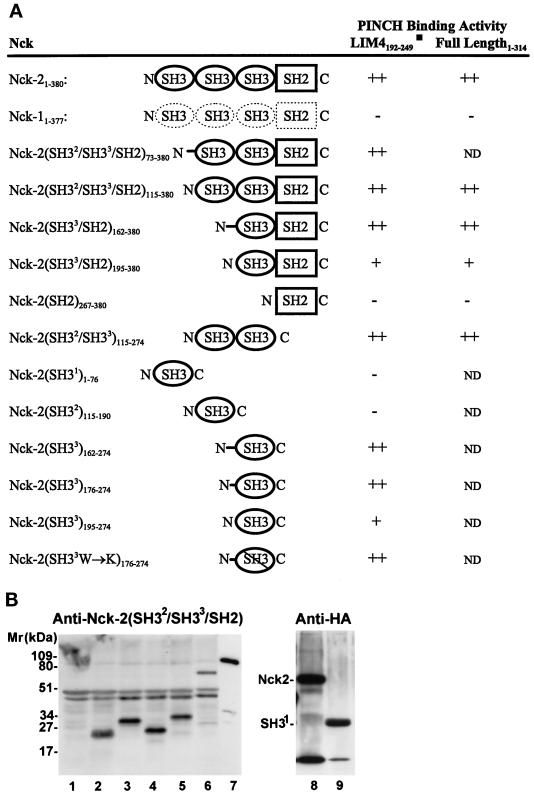
Figure 3.
Mapping of the PINCH binding site on Nck-2. The cDNA sequences encoding the full-length or various domains of Nck-2 and Nck-1 were inserted into the pB42AD vector. The cDNAs encoding the full-length PINCH or its LIM4 domain were inserted into the pLexA vector. (A) PINCH binding activity. The numbers in subscript indicate amino acid residues of Nck-2, Nck-1, and PINCH encoded by each construct. W234→K, the conserved W (amino acid residue 234) in the third SH3 domain of Nck-2 was changed to K. The PINCH binding activity was determined based on the interaction of each of the Nck proteins with the LIM4 domain and/or the full length PINCH in yeast two-hybrid assays as described in MATERIALS AND METHODS. ++, Growth of blue colonies on the leucine-deficient selection medium containing 80 μg/ml X-gal was detected within 1 d; +, growth of blue colonies on the leucine-deficient selection medium containing 80 μg/ml X-gal was detected within 2 or 3 d; −, no blue colony was detected after 5 d. ND, not determined. (B) Expression of B42AD fusion proteins containing various Nck-2 and Nck-1 sequences. The expression of the Nck fusion proteins in yeast cells harboring the pLexA-LIM4 and the pB42AD that contains the first SH3 domain of Nck-2 (residues 1–76)(lanes 1 and 9), the second SH3 domain of Nck-2 (residues 115–190)(lane 2), the third SH3 domain of Nck-2 (long form, residues 176–274)(lane 3), the third SH3 domain of Nck-2 (short form, residues 195–274)(lane 4), the SH2 domain of Nck-2 (residues 267–380)(lane 5), the full- length Nck-1 (lane 6), or the full-length Nck-2 (lane 8) were determined by immunoblotting. Yeast cells from 3-ml cultures were extracted with 200 μl of urea/SDS protein extraction buffer (40 mM Tris-HCl, pH 6.8, containing 8 M urea, 5% (wt/vol) SDS, 0.1 mM EDTA, and 0.4 mg/ml) and lanes 1–6, 8, and 9 were loaded with 20 μl of the yeast extracts per lane. Lane 7 was loaded with 1 ng MBP fusion protein containing the full-length Nck-2. Lanes 1–7 were probed with a rabbit anti-Nck antibody raised against a GST fusion protein containing the two C-terminal SH3 domains and the SH2 domain of Nck-2 (residues 115–380), and lanes 8 and 9 were probed with a rabbit anti-HA antibody (Zymed Laboratories; 1 μg/ml).