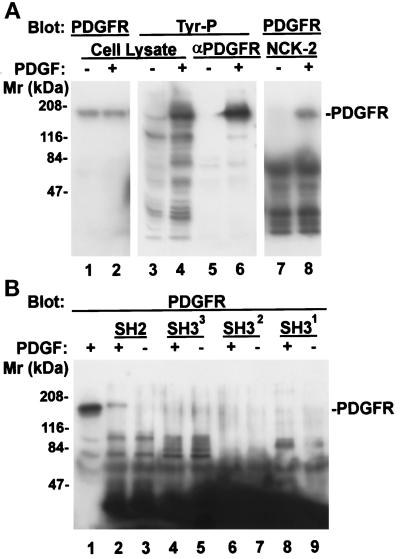
Figure 6.
Association of Nck-2 with PDGF receptor-β. NIH3T3 cell monolayers (∼80% confluent) were starved in DMEM containing 0.2% FBS for 18 h. At the end of serum starvation, the cells were either harvested (−PDGF), or stimulated with 50 ng/ml PDGF (+PDGF) for 5 min and then lysed. The PDGF receptor-β was precipitated from the cell lysates (1 ml of 2.8 mg protein/ml) with 1 μg of rabbit polyclonal anti-PDGF receptor-β antibody (P-20, Santa Cruz Biotechnology) and protein G-Sepharose 4B beads (panel A, lanes 5 and 6, one half of the immunoprecipitates/lane). The protein tyrosine phosphorylation was determined by immunoblotting with a mouse monoclonal anti-phosphotyrosine antibody (PY20, 0.2 μg/ml) (panel A, lanes 3–6). For GST fusion protein pull-down experiments, the cell lysates (0.37 mg protein) were incubated with 10 μg of the GST fusion protein containing the full-length Nck-2 (panel A, lanes 7 and 8) or its individual SH domains (panel B, lanes 2–9) as indicated in the figure (final volume = 220 μl). The GST fusion proteins were precipitated with glutathione-Sepharose beads. The PDGF receptor-β that was associated with the Nck-2 proteins (panel A, lanes 7 and 8; panel B, lanes 1–9) or those in the total cell lysates (panel A, lanes 1 and 2) were detected with a rabbit anti-PDGF receptor-β antibody (P-20, 1 μg/ml). Lanes 1–4 in panel A and lane 1 in panel B were loaded with cell lysates (21 μg protein/lane).