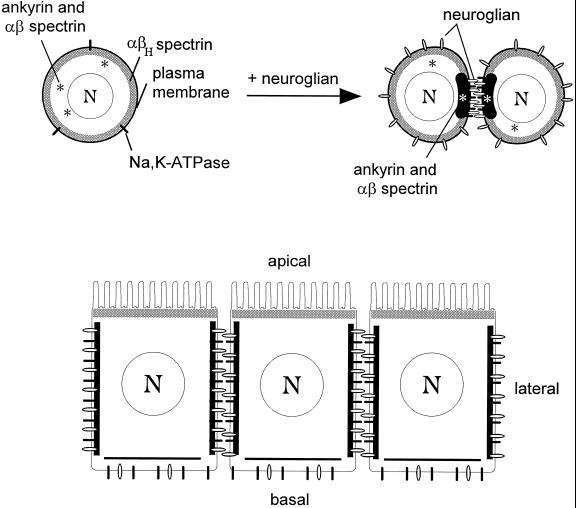
Figure 7.
Segregation of spectrin isoforms in response to positional cues at the cell surface. (A) The uniform distribution of αβH spectrin (submembrane shading) at the plasma membrane of S2 cells (left) implies the presence of a constitutive βH-specific receptor that is uniformly distributed at the cell surface. In contrast, ankyrin and αβ spectrin are found in the cytoplasm of control S2 cells (*, left), but they are recruited to sites of cell–cell contact in response to neuroglian-mediated cell adhesion (right). The Na,K-ATPase also becomes concentrated at cell contacts in response to neuroglian expression and ankyrin/αβ spectrin assembly. (B) Neuroglian, ankyrin, αβ spectrin, and Na,K-ATPase are concentrated at sites of cell–cell contact in the larval salivary gland epithelium, in agreement with the order of events observed in S2 cells. However, the putative αβH spectrin receptor is sorted apically in these polarized cells. Despite their common features (including an identical α subunit), the two spectrin isoforms respond independently to their respective membrane receptors to form segregated membrane skeleton domains.