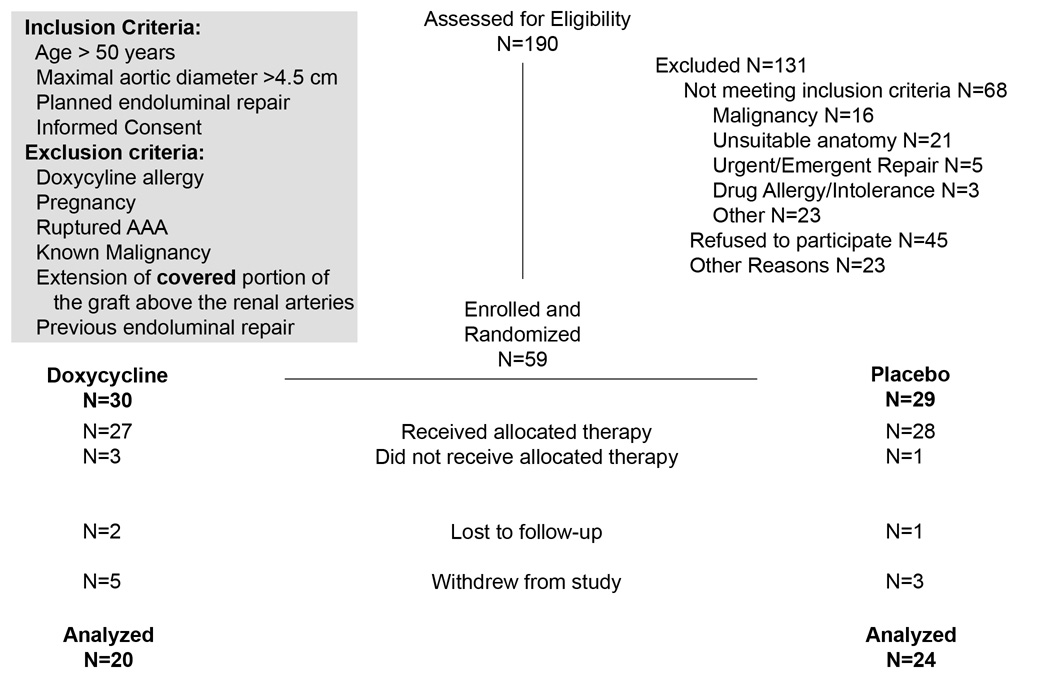
Figure 1. Study enrollment data.
Inclusion and exclusion criteria for enrollment are as listed at the conclusion of the study. During the first year of enrollment, the maximal aortic diameter necessary for enrollment was 5.0 cm; this resulted in most of the anatomic exclusions. After randomization, but before dispensing the study medications we had 4 withdrawals: 1 developed a postoperative ileus and was unable to take the medication. 1 patient had an open AAA repair, and 1 patient from each group decided not to accept the study medications after surgery and withdrew. After the medications were dispensed we had an additional 8 withdrawals before 6 months of follow-up, 1 Doxy and 1 Placebo patients withdrew claiming medication GI side effects, 4 developed other illnesses and declined further participation, and 2 withdrew without stating a reason, 1 person withdrew due to a change in insurance coverage and did not return to our facility, 1 patient in the placebo group died from complications of COPD and there was 1 patient whom we could not contact and was lost to subsequent follow-up.