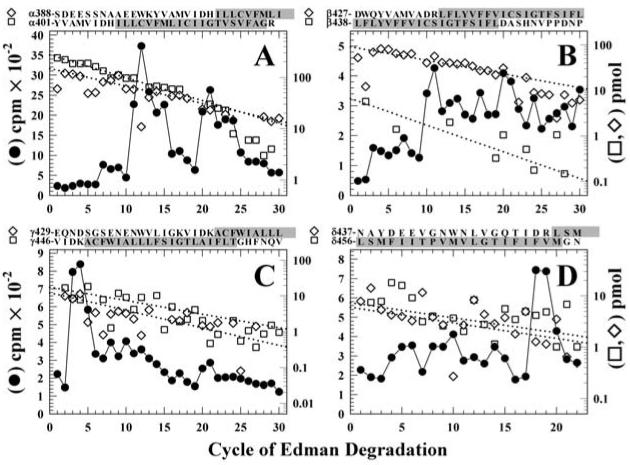
Figure 6. Sequence analysis of [3H]Azicholesterol labeled fragments containing the M4 segment.
Peaks of 3H from the HPLC fractionations of tryptic digests of nAChR subunit fragments (Figure 5) were pooled for sequencing. Panel A, the digest of αV8-10. Fragments were detected beginning at αTyr-401 (□, Io, 274 pmol; R, 90%) and αSer-388 (◇, Io, 148 pmol; R, 92.5%) (254,000 cpm loaded on the filter and 25,600 cpm remaining after 30 cycles), with other unidentified peptides present at <10 pmol. Panel B, the digest of βV8-12. The primary sequence began at βAsp-427 (◇, Io, 86 pmol; R, 94%), with secondary sequences beginning at βLeu-438 (□, Io, 9 pmol; R, 91%), βLys-216 (βM1, Io, 9 pmol; R, 94%, not shown), and βMet-249 (βM2, Io, 4 pmol; R, 92%, not shown) (90,000 cpm loaded, 5,500 cpm remaining after 40 cycles). Panel C, the trypsin digest of γV8-14. Four fragments were present beginning at γVal-446 (□, Io, 18 pmol; R, 92%), γGlu-429 (◇, Io, 12 pmol; R, 89%), γThr-276 (γM3, Io, 28 pmol; R, 90%, not shown), and γLys-218 (γM1, ∼5 pmol) with (53,000 cpm loaded on the filter, 3,300 cpm remaining after 30 cycles). Panel D, the trypsin digest of δV8-11. Peptides were identified beginning at δAsn-437 (□, Io, 6 pmol; R, 93%), δLeu-456 (◇, Io, 7 pmol; R, 93%), δLys-224 (δM1, Io, 12 pmol; R, 94%, not shown), δMet-257 (δM2, Io, 17 pmol; R, 89%, not shown), and Thr-28 from the β-subunit of the Na+/K+-ATPase (Io, 10 pmol; R, 96%, not shown) (90,000 cpm loaded on the filter, 5,500 cpm remaining after 22 cycles). For each sample, ∼83% of each cycle of Edman degradation was analyzed for released 3H (●) and ∼17% for PTH-derivatives (□,◇), with the dotted line corresponding to the fit of the amount of detected PTH-derivatives. The amino acid sequences of the fragments containing the M4 segments are shown above each panel, with the limits of the M4 regions shaded.