Figure 7. V8 protease digestion of [3H]Azicholesterol labeled V8 protease fragments αV8-20, βV8-22, γV8-24, and δV8-20.
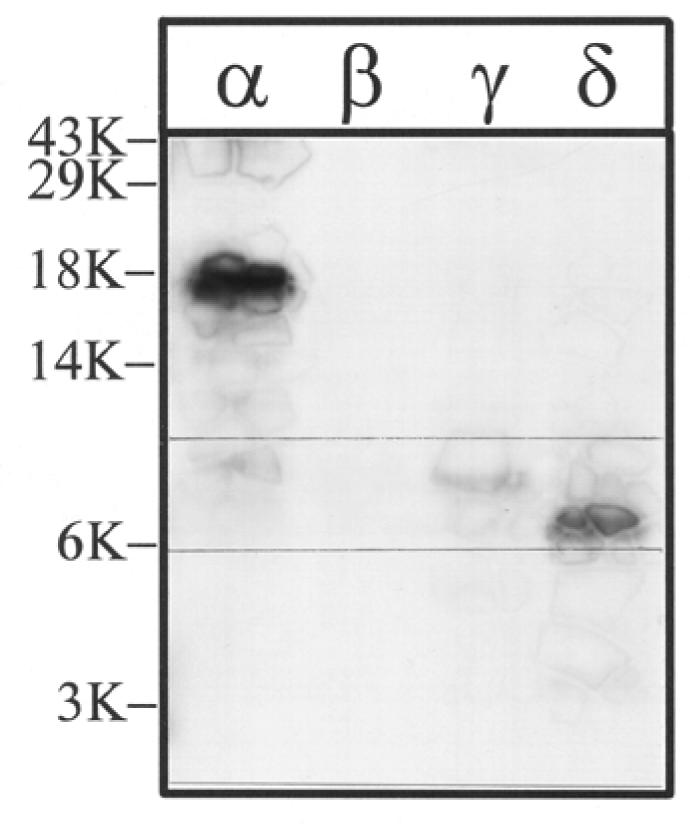
The V8 protease fragments αV8-20, βV8-22, γV8-24, and δV8-20, isolated from nAChRs labeled with 1.25 μM [3H]Azicholesterol in the presence of agonist, were exhaustively digested with S.aureus V8 protease (400% w/w) for six days. Aliquots of the total digests (∼10%) were fractionated by Tricine SDS-PAGE and then subjected to fluorography for 12 weeks. The migration of prestained molecular weight standards are indicated on the left. For each digest, there is a principal band of 3H which migrates with apparent molecular masses of: ∼ 8.5 kDa (α), 7 kDa (β), 7.5 kDa (γ), and 7.5 kDa (δ) (bracketed gel region). For the digest of αV8-20 there remains a substantial amount of undigested material and this band has approximately the same electrophoretic mobility as the 18 kDa standard.
