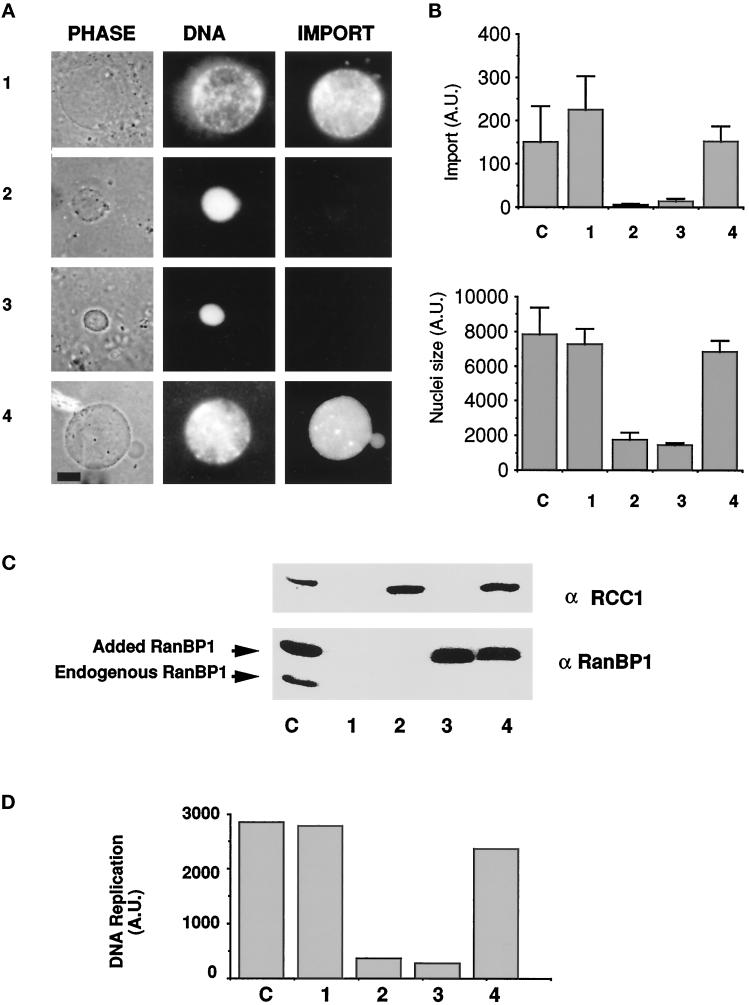
Figure 6.
Balance of RCC1 and RanBP1 is critical for nuclear assembly and function. (A) Imbalance of RanBP1 and RCC1 causes defects in nuclear assembly and protein import. Equal volumes of XB Buffer (row 1), recombinant RCC1 (final concentration = 10 μg/ml; row 2), recombinant RanBP1 (final concentration = 50–70 μg/ml; row 3), or both RCC1 and RanBP1 (row 4) were added to codepleted cytosol and incubated for 15 min on ice. The cytosol was then used in nuclear assembly reactions. A rhodamine-labeled import substrate was added 60 min after nuclear assembly began, and 120 min after the reaction began, each sample was assayed for nuclear morphology and protein import. Phase-contrast images of typical nuclei are shown on the left; corresponding photographs of nuclear DNA (stained with Hoechst 33258) and protein import assays are shown in the middle and right, respectively. Bar, 2.6 × 10−6 m. [As a control, BSA was added to codepleted extracts. BSA neither inhibited nuclear assembly in codepleted extracts nor restored nuclear assembly in codepleted extracts to which either RanBP1 or RCC1 had been added (our unpublished results).] (B) Quantitation of protein import and nuclear size among control and experimental samples. A set of samples similar to those in A (bars 1–4) plus a control (bar C) was allowed to form nuclei under the conditions described above. After 90 min, images of at least 25 nuclei from each sample were randomly selected and captured by using an IP Labs Spectrum Imaging System with a Photometrics cooled charge-coupled device camera. Capture was performed under identical conditions for each sample and the intensity of the signal was not saturating the imaging system. Nuclear size was measured as the number of pixels occupied by the nucleus at its maximal cross-sectional area. Import was measured as pixel intensity within the nucleus, corrected for background fluorescence. The mean and standard deviations were calculated to obtain the relative levels of import (top) and nuclear size (bottom). (C) RanBP1 and RCC1 protein levels. A 1-μl volume from each of the nuclear assembly reactions shown in A was subjected to SDS-PAGE and Western blot analysis with anti-RCC1 or anti-Xenopus RanBP1 antibodies, as indicated. Lane C shows the amount and positions of endogenous RCC1, RanBP1, and added recombinant RanBP1 (bigger than endogenous RanBP1 because of the tag). (D) DNA replication is inhibited by the addition of either RCC1 or RanBP1 but restored by the addition of both. Reactions of a control extract and those shown in A were allowed to undergo DNA replication in the presence of [α-32P]dCTP. At 180 min, samples from the control (bar C) and reactions of codepleted extract containing XB buffer (bar 1), recombinant RCC1 (bar 2), recombinant RanBP1 (bar 3), or both RCC1 and RanBP1 (bar 4) were taken for analysis of 32P incorporation. The samples were treated as previously described (Smythe and Newport, 1991) and the amount of 32P incorporated into high molecular weight DNA was quantified using a Phosphorimager.