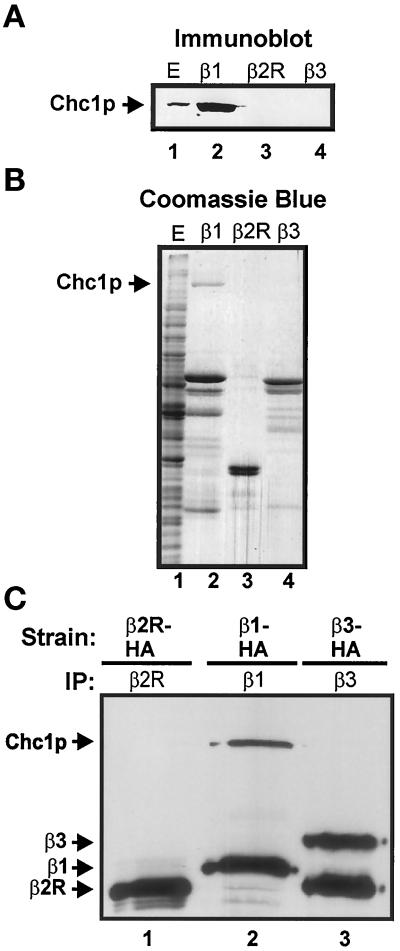
Figure 2.
AP-1 interacts with clathrin. (A and B) C-terminal regions of β sbunits were fused to GST and expressed in E. coli. Fusion proteins were bound to glutathione-Sepharose beads and then incubated with extract from strain TVY 614. Bound proteins were eluted and separated by SDS-PAGE. Proteins were transferred to nitrocellulose and immunoblotted with antibodies to detect Chc1p (A) or stained with Coomassie brilliant blue (B). Lane 1 in both panels contains a sample of the starting extract corresponding to 1/10,000 of the extract used for incubations with GST fusions. The most prominent species in lanes 2–4 are the GST fusion proteins. (C) Immunoprecipitations of AP β subunits. Polyclonal antibodies were used to precipitate Apl1p (β2R; lane 1), Apl2p (β1; lane 2), or Apl6p (β3; lane 3) from native extracts of Apl1p(β2R)-HA-expressing (GPY 2109), Apl2p(β1)-HA-expressing (GPY 2110), or Apl6p(β3)-HA-expressing (GPY 2171) strains. Precipitates were subjected to SDS-PAGE, transferred to nitrocellulose, and immunblotted with monoclonal antibodies to detect Chc1p and HA epitopes. The lower-molecular-weight band in lane 3 is a β3 degradation product.