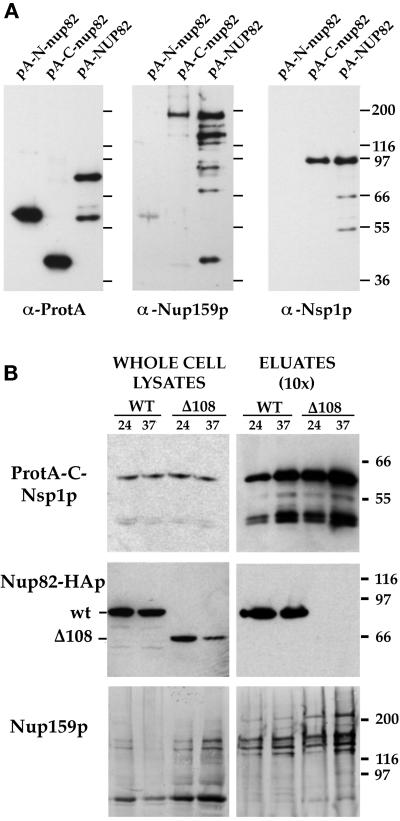
Figure 3.
Nsp1p and Nup159p form a core complex independent of interaction with the carboxyl-terminal domain of Nup82p. (A) Affinity purification by IgG-Sepharose chromatography of ProtA-N-nup82p, ProtA-C-nup82p, and ProtA-Nup82p expressed in strains derived from BJ2168. The purified fractions were analyzed by Western blotting using IgG coupled to HRP to detect the ProtA fusions, an anti-Nup159p antibody directed against the carboxyl-terminal domain of Nup159p (rat7#5), and an antibody directed against the repeat domain of Nsp1p. (B) Whole-cell lysates from NUP82 (WT) and nup82-Δ108 (Δ108) strains transformed with the pRS316-ProtA-C-Nsp1p plasmid and maintained at 24°C or shifted to 37°C for 3 h were affinity purified by IgG-Sepharose chromatography. Whole-cell lysates and affinity-purified fractions (eluates, 10-fold equivalent) were analyzed by Western blotting for the presence of ProtA-C-Nsp1p using an anti-IgG coupled to HRP, for the presence of Nup82-HAp and Nup82-Δ108-HAp with a monoclonal antibody directed against the HA epitope, and for the presence ofNup159p with the rat7#4 antibody. Although Nup82-Δ108-HAp does not interact with ProtA-C-Nsp1p at either 24 or 37°C, Nup159p still copurifies with ProtA-C-Nsp1p in the nup82Δ108 strain. Note that the NUP82-wt and nup82Δ108 strains are not protease deficient, leading to a substantial degradation of Nup159p. The shorter degradation product of Nup159p did not copurify with ProtA-C-Nsp1p.