Abstract
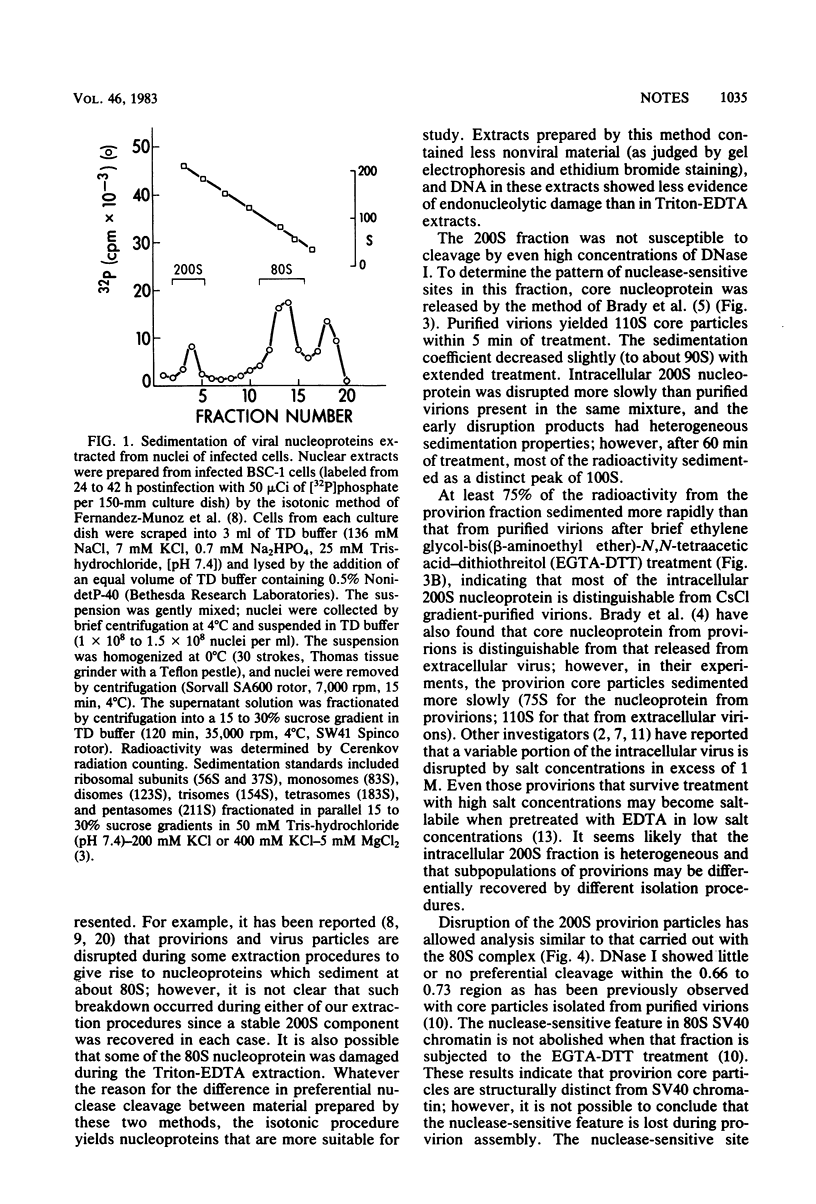
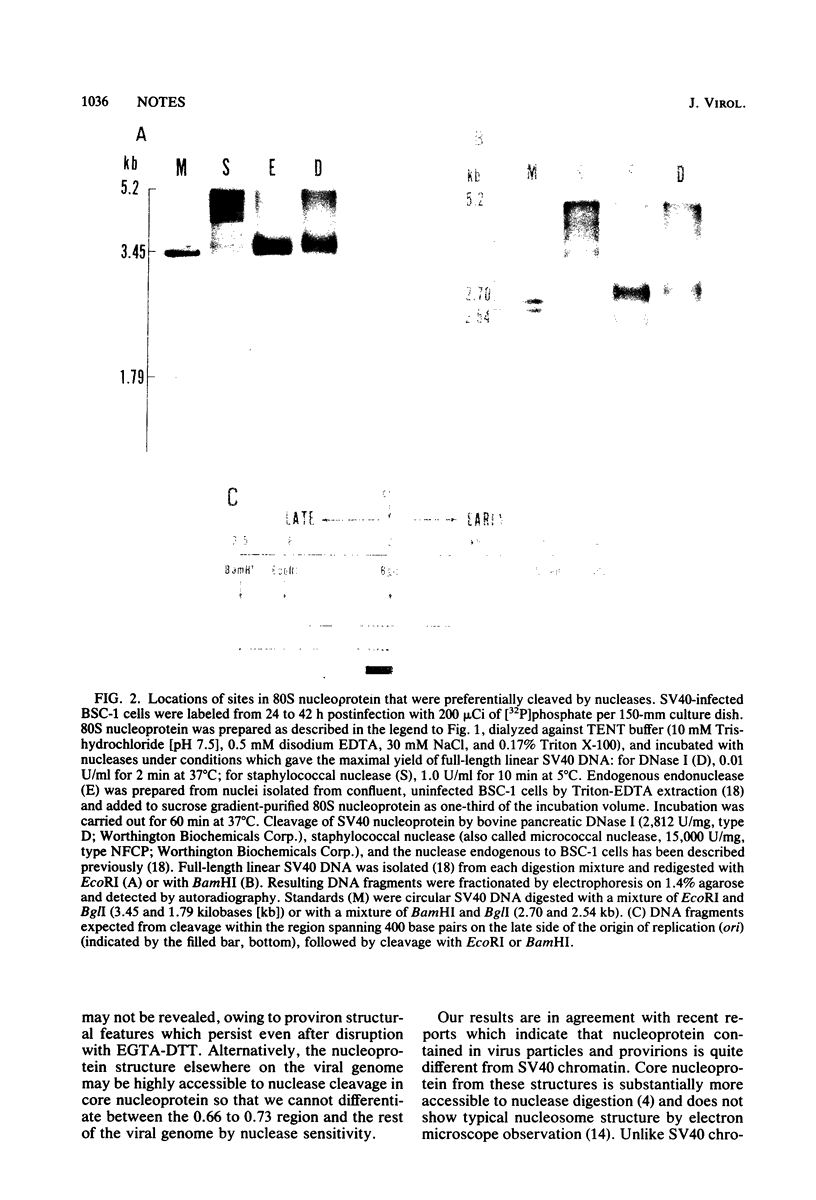
Simian virus 40 nucleoprotein isolated from the nuclei of infected cells contains a nuclease-sensitive site adjacent to the viral origin of replication (between 0.66 and 0.73 map unit). Nuclear extracts were subfractionated by sucrose gradient centrifugation to yield provirions (200S) and simian virus 40 chromatin (80S). The 80S fraction was cleaved either by DNase I or by an endonuclease endogenous to BSC-1 cells with high preference for the 0.66 to 0.73 region. The 200S fraction was treated to release core particles that were sensitive to nuclease cleavage; however, DNase I showed little or no preference for the 0.66 to 0.73 region of the provirion core nucleoprotein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakayev V. V., Nedospasov S. A. SV40-specific nucleoprotein complexes: heterogeneity and composition. Virology. 1981 Mar;109(2):244–256. doi: 10.1016/0042-6822(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Baumgartner I., Kuhn C., Fanning E. Identification and characterization of fast-sedimenting SV40 nucleoprotein complexes. Virology. 1979 Jul 15;96(1):54–63. doi: 10.1016/0042-6822(79)90172-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Radonovich M., Lavialle C., Salzman N. P. Simian virus 40 maturation: chromatin modifications increase the accessibility of viral DNA to nuclease and RNA polymerase. J Virol. 1981 Aug;39(2):603–611. doi: 10.1128/jvi.39.2.603-611.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Prados M., Yu H. Y., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. IV. Micrococcal nuclease digestion. J Virol. 1982 Nov;44(2):603–609. doi: 10.1128/jvi.44.2.603-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Baumgartner I. Role of fast-sedimenting SV40 nucleoprotein complexes in virus assembly. Virology. 1980 Apr 15;102(1):1–12. doi: 10.1016/0042-6822(80)90064-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. I. Methods of isolation and characterization in CV-1 cells. J Virol. 1979 Feb;29(2):612–623. doi: 10.1128/jvi.29.2.612-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. The detection and characterization of multiple forms of SV40 nucleoprotein complexes. Virology. 1978 Oct 15;90(2):305–316. doi: 10.1016/0042-6822(78)90315-x. [DOI] [PubMed] [Google Scholar]
- Hartmann J. P., Scott W. A. Distribution of DNase I-sensitive sites in simian virus 40 nucleoprotein complexes from disrupted virus particles. J Virol. 1981 Mar;37(3):908–915. doi: 10.1128/jvi.37.3.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Aloni Y. Isolation and characterization of various forms of simian virus 40 DNA-protein complexes. Virology. 1980 Apr 15;102(1):107–118. doi: 10.1016/0042-6822(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Bratosin S., Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- La Bella F., Vesco C. Late modifications of simian virus 40 chromatin during the lytic cycle occur in an immature form of virion. J Virol. 1980 Mar;33(3):1138–1150. doi: 10.1128/jvi.33.3.1138-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyne G., Harper F., Saragosti S., Yaniv M. Absence of nucleosomes in a histone-containing nucleoprotein complex obtained by dissociation of purified SV40 virions. Cell. 1982 Aug;30(1):123–130. doi: 10.1016/0092-8674(82)90018-6. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Georgiev G. P. Non-random cleavage of SV40 DNA in the compact minichromosome and free in solution by micrococcal nuclease. Biochem Biophys Res Commun. 1980 Jan 29;92(2):532–539. doi: 10.1016/0006-291x(80)90366-6. [DOI] [PubMed] [Google Scholar]
- Robinson G. W., Hallick L. M. Mapping the in vivo arrangement of nucleosomes on simian virus 40 chromatin by the photoaddition of radioactive hydroxymethyltrimethylpsoralen. J Virol. 1982 Jan;41(1):78–87. doi: 10.1128/jvi.41.1.78-87.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Seidman M., Garber E., Levine A. J. Parameters affecting the stability of SV40 virions during the extraction of nucleoprotein complexes. Virology. 1979 May;95(1):256–259. doi: 10.1016/0042-6822(79)90427-6. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Staphylococcal nuclease makes a single non-random cut in the simian virus 40 viral minichromosome. J Mol Biol. 1979 Aug 15;132(3):535–546. doi: 10.1016/0022-2836(79)90274-2. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Föhring B., Chowdhury K., Gruss P., Sauer G. Origin of DNA replication in papovavirus chromatin is recognized by endogenous endonuclease. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5964–5968. doi: 10.1073/pnas.75.12.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]