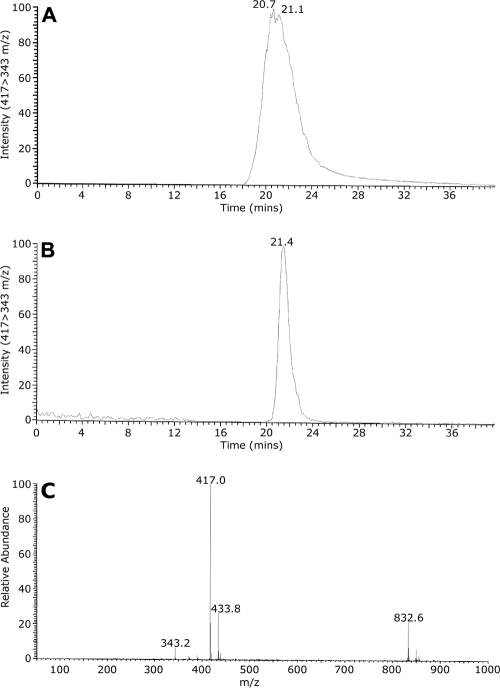
FIG. 3.
Reconstitution of the N,N′-bisformyl dityrosine synthase catalytic activity of C. albicans CYP56. N,N′-Bisformyl dityrosine synthase activity assays were performed using 10 μM N-formyl tyrosine as the substrate. The assay product, N,N′-bisformyl dityrosine, was isolated by solvent extraction and TLC prior to analysis by LC-MS-MS along with a control horseradish peroxidase-synthesized sample of N,N′-bisformyl dityrosine. (A and B) N,N′-Bisformyl dityrosine was identified in the C18 column eluates of the control (A) and reconstitution assay (B) samples by MS-MS, with continuous monitoring of the relative abundance of the SRM transition from 417 to 343 m/z. (C) Full-scan MS of N,N′-bisformyl dityrosine confirmed the molecular mass ion to be 417 m/z. Other abundant mass ions present included a monohydroxylated N,N′-bisformyl dityrosine at 433.8 m/z and the molecular mass ion of formyl-tetratyrosine at 832.6 m/z.