Abstract
Tsetse flies (Diptera: Glossinidae) are vectors for trypanosome parasites, the agents of the deadly sleeping sickness disease in Africa. Tsetse also harbor two maternally transmitted enteric mutualist endosymbionts: the primary intracellular obligate Wigglesworthia glossinidia and the secondary commensal Sodalis glossinidius. Both endosymbionts are transmitted to the intrauterine progeny through the milk gland secretions of the viviparous female. We administered various antibiotics either continuously by per os supplementation of the host blood meal diet or discretely by hemocoelic injections into fertile females in an effort to selectively eliminate the symbionts to study their individual functions. A symbiont-specific PCR amplification assay and fluorescence in situ hybridization analysis were used to evaluate symbiont infection outcomes. Tetracycline and rifampin treatments eliminated all tsetse symbionts but reduced the fecundity of the treated females. Ampicillin treatments did not affect the intracellular Wigglesworthia localized in the bacteriome organ and retained female fecundity. The resulting progeny of ampicillin-treated females, however, lacked Wigglesworthia but still harbored the commensal Sodalis. Our results confirm the presence of two physiologically distinct Wigglesworthia populations: the bacteriome-localized Wigglesworthia involved with nutritional symbiosis and free-living Wigglesworthia in the milk gland organ responsible for maternal transmission to the progeny. We evaluated the reproductive fitness, longevity, digestion, and vectorial competence of flies that were devoid of Wigglesworthia. The absence of Wigglesworthia completely abolished the fertility of females but not that of males. Both the male and female Wigglesworthia-free adult progeny displayed longevity costs and were significantly compromised in their blood meal digestion ability. Finally, while the vectorial competence of the young newly hatched adults without Wigglesworthia was comparable to that of their wild-type counterparts, older flies displayed higher susceptibility to trypanosome infections, indicating a role for the mutualistic symbiosis in host immunobiology. The ability to rear adult tsetse that lack the obligate Wigglesworthia endosymbionts will now enable functional investigations into this ancient symbiosis.
Human African trypanosomiasis is a fatal disease caused by the protozoan Trypanosoma brucei and transmitted by the tsetse fly (Diptera: Glossinidae). Antigenic variation displayed by the parasite in the mammalian host has hampered vaccine development efforts. Disease control further suffers from lack of cheap and efficacious drugs and practical diagnostic tools. Reducing the tsetse fly population is known to be effective in the elimination of the disease, and expanding the repertoire of vector-based control tools is a high priority. Because tsetse feed exclusively on vertebrate blood, they have established symbiotic associations with microbes, on which they rely for supplementing their restricted diet with nutrients they cannot produce. Hence, these symbiotic associations provide a weak link in the fly's biology and can be further exploited for developing novel biological control methods. Two of the symbionts are enteric in nature: the obligate mutualist species Wigglesworthia glossinidia (2) and the facultative mutualist species Sodalis glossinidius (10). In addition, many tsetse populations harbor a parasitic microbe belonging to the genus Wolbachia. It has been difficult to study the individual functions of the multiple symbionts in host physiology, as attempts to cure tsetse of Wigglesworthia infections were found to result in sterility.
Wigglesworthia resides within the cytoplasm of the differentiated epithelial cells, bacteriocytes which form the bacteriome organ in the adult anterior midgut. Phylogenetic studies have shown that association between the tsetse hosts and their Wigglesworthia symbiont is ancient (50 to 80 million years old) and displays concordance (7). Similar to other insect mutualists, Wigglesworthia has a highly streamlined genome of about 700 kb (1). The presence of Wigglesworthia has been shown to be essential for host fecundity, as its elimination by antibiotics has been shown to render females sterile (27). Provisioning the host blood meal diet with antibiotics supplemented with a vitamin cocktail has been shown to rescue sterility partially—implying a role for Wigglesworthia in vitamin supplementation (26). Further evidence of a role for Wigglesworthia in vitamin provisioning comes from the genome data, which show that the small genome of Wigglesworthia has retained 62 genes involved in the biosynthesis of various cofactors, prosthetic groups, and carriers (1). Association with primary endosymbionts for host nutrient provisioning is common in insects with restricted nutritional ecologies. A role for host metabolic supplementation has also been suggested for the primary symbionts of aphids (Buchnera aphidicola), where the small genome of this symbiont encodes all of the essential amino acids, which are apparently low in the host diet (18). Comparison of the putative proteomes of Buchnera in aphids, Blochmannia in ants, and Wigglesworthia in tsetse has revealed a metabolic interdependence between these symbionts and their corresponding hosts and is suggestive of a nutritional symbiosis for each association (42).
The second tsetse symbiont, the facultative mutualist Sodalis, resides intra- and extracellularly in the midgut tissue and can also be detected in the hemolymph (8). Its sequenced genome of 4.2 Mb reveals features typically associated with closely related free-living enterics (34). Sodalis' genome, however, has only 49% coding capacity and carries an unusually high number of pseudogenes, presumably reflecting an ongoing erosion process as the organism adapts from a free-living to a symbiotic lifestyle (34). Unlike other insect symbionts such as the intracellular Wigglesworthia and Wolbachia bacteria, which cannot be cultivated in vitro, outside their host cells, Sodalis bacteria can be grown axenically, apparently again indicative of their relatively recent symbiotic status in tsetse. The functional role of Sodalis symbiosis in tsetse is relatively unknown, but in one study its elimination via the metabolic inhibitor streptozotoicin has been associated with decreased longevity in the progeny (11). In addition, Sodalis has been implicated in the host's ability to establish trypanosome infections (36, 38).
It has been difficult to study the physiological roles of tsetse symbionts, as antibiotic treatment of fertile flies results in the elimination of Wigglesworthia and induces host sterility, making it difficult to generate fly lines carrying single symbionts. In the aphid system, it has been possible to differentially clear either the primary or the secondary symbionts by using intrahemocoelic injections of different antibiotics. In studies where the primary symbionts have been cleared, it has been shown that pea aphid secondary symbionts can compensate for the essential role of the primary symbiont Buchnera, which enables host survival and reproduction (18). Other secondary facultative symbionts such as pea aphid U-type symbionts have been shown to confer a fitness advantage on their host such as host plant specialization (35), and the pea aphid secondary symbiont (T type as well as R type) is known to mediate resistance against parasitoid wasps (28) and against environmental temperature shifts (24, 30). Generally, in aphids, the presence of the secondary symbiont is associated with a suppressive or negative effect on the primary symbionts with fitness cost to the host (18, 32). These studies illuminate the complex symbiont-symbiont and host-symbiont interactions in endosymbiotic insect systems (18, 35).
Lastly, some tsetse populations harbor a third microbe which is a member of the genus Wolbachia, a rickettsia-like parasitic bacterium found in numerous arthropods (17, 39, 40). Wolbachia infections can result in a wide range of phenotypic effects in arthropods, such as host reproductive alterations, premature death, and behavioral modifications (reviewed in reference 14). The tissue distribution and prevalence of Wolbachia in tsetse species range from intracellular infection of only germ line tissue to persistence in various somatic tissues (9, 12). Attempts to cure Wolbachia infections through antibiotic administration have similarly not been possible as these treatments result in host sterility.
Tsetse have an unusual viviparous reproductive biology (bearing live young) and typically produce 8 to 10 offspring in their life span. To carry out this life cycle, tsetse reproductive physiology has undergone extensive physiological (and morphological) modifications. These modifications include a reduced number of ovarioles per ovary (two), development of a highly tracheated and muscular uterus, and modification of the accessory gland into an organ that supplies nutrients to the developing larvae (milk gland). The larva produced by hatching of a single egg at a time develops in utero and receives nutritional supplements from the mother's milk glands in the form of milk secretions rich in protein and lipid. After a period of maturation and sequential moulting within the mother, the third-instar larva is deposited and pupates shortly thereafter (21). By using a species-specific PCR amplification assay, Sodalis has been detected in the milk gland organ and thus may be transmitted to the progeny via the milk gland secretions (8). The presence of large bacteria reminiscent of Wigglesworthia has also been observed by microscopy in the milk gland lumen (22). Recent fluorescent in situ hybridization (FISH) analysis with species-specific oligonucleotide probes has shown the presence of both Sodalis and Wigglesworthia in the milk gland lumen and some intracellular Sodalis infections with the milk gland cells (5).
The overall objective of this study was to understand the individual functional role(s) of the tsetse fly's mutualistic flora in host fitness. In this analysis, we supplemented the host blood diet continuously, as well as discretely, via hemocoelic injections with several different antibiotics with known modes of action on intracellular and free-living microbes. We report on the selective elimination of the primary symbiont Wigglesworthia from tsetse progeny and on the putative effects of the Wigglesworthia symbiosis on a variety of host fitness parameters, including fecundity, longevity, digestion, and vector competence.
MATERIALS AND METHODS
Insects.
The Glossina morsitans subsp. morsitans colony maintained in the insectary at Yale University was originally established from puparia from fly populations in Zimbabwe. Newly emerged flies are separated by sex and mated at 3 to 4 days posteclosion. Flies are maintained at 24 ± 1°C and 50 to 55% relative humidity and receive defibrinated bovine blood every 48 h by an artificial membrane system (23).
Antibiotic treatments.
Newly eclosed adult female G. morsitans subsp. morsitans flies (numbers are shown in Table 1) were divided into 12 groups, 8 of which were provided with either normal (control) blood meals or blood meals supplemented with ampicillin at 30 μg/ml, ampicillin at 50 μg/ml, carbenicillin at 30 μg/ml, tetracycline at 25 μg/ml, rifampin at 20 ng/ml, or rifampin at 60 ng/ml every 2 days for 60 days. Two groups received either ampicillin or carbenicillin (1 μg/mg of body weight) treatments by intrathoracic microinjection into the hemolymph as teneral (newly emerged) flies, and two more groups received an additional injection as 10-day-old adults. The experiments were conducted in three replicates.
TABLE 1.
Fecundity outcomes of groups of flies maintained under different antibiotic treatments
| Treatment | No. of females (total no. of puparia deposited)b | Fecundity index (no. of pupae/female) | Relative hatching rate (%)c | Reproductive status
|
|
|---|---|---|---|---|---|
| F1 | F2 | ||||
| Control | 20 (51) | 2.55 | 100 | Fertile | Fertile |
| Ampicillin per os (30 μg/ml) | 16 (40) | 2.5 | 78 | Sterile | |
| Ampicillin per os (50 μg/ml) | 50 (93) | 1.86 | 79 | Sterile | |
| Tetracycline per os (25 μg/ml) | 25 (14) | 0.56 | Sterile | ||
| Rifampin per os (60 μg/ml) | 25 (24) | 0.96 | Sterile | ||
| Rifampin per os (20 μg/ml) | 20 (15) | 0.75 | Sterile | ||
| Carbenicillin per os (30 μg/ml) | 30 (60) | 2 | 86 | Sterile | |
| Carbenicillin per os (30 μg/ml) on alternate days | 50 (115) | 2.3 | 78 | Steriled | |
| Ampicillin injection oncea | 10 (35) | 3.5 | 93 | Sterile | |
| Ampicillin injection twicea | 11 (36) | 3.2 | 100 | Sterile | |
| Carbenicillin injection oncea | 11 (36) | 3.2 | 94 | Fertile | Fertile |
| Carbenicillin injection twicea | 36 (92) | 2.55 | 100 | Fertile | Fertile |
Total dosage of 1 μg/mg body weight.
Puparia were collected over three gonotrophic cycles.
The hatching rate is expressed as a percentage of that observed in the control treatment group.
With the exception of the first gonotrophic cycle adults, which were fertile.
Monitoring the fecundity cost of antibiotic treatments.
G. morsitans subsp. morsitans females were mated after receiving two blood meals, and copulation was observed. Females were separated and monitored for three gonotrophic cycles (approximately 51 days) in each respective treatment group as described above. The mortality rate and the number of pupae deposited by each group were recorded during this period. Fecundity is expressed as the number of pupae deposited per female for each of the three gonotrophic cycles. The pupae from each group were allowed to hatch, the female progeny was mated with normal untreated males, and copulation was observed for all crosses. All progeny received normal blood meals, and their fecundity was similarly monitored.
Symbiont quantification. (i) DNA preparation from fly tissues.
The microscopically dissected guts and remaining abdomens (carcasses) of 30-day-old per os antibiotic-treated mothers and 60-day-old mothers that received hemocoelic antibiotic injections were collected. The progeny used for tissue collections were 7 to 10 days old after eclosion. Gut samples were used to quantify the bacteriome-localized Wigglesworthia and Sodalis bacteria present in the gut tissue. The entire gut was dissected immediately posterior to the proventriculus, and the remaining carcass devoid of thorax was used to analyze the symbionts in accessory milk gland tissue. Dissected tissues were flash frozen in liquid nitrogen and kept at −20°C until processing. Total DNA was extracted by the method of Holmes and Bonner (15) and quantified by spectrophotometer. A minimum of three gut and carcass samples was analyzed from each group. The concentration of the DNA was determined by spectrophotometer measurements.
(ii) Diagnostic PCR assay.
The presence of Sodalis and Wigglesworthia was determined by using a symbiont species-specific PCR amplification assay. Four nanograms of template genomic DNA was used for each PCR. Prior titration of template concentrations over 30 PCR cycles determined that a 4-ng template DNA was in the linear range of the density of the PCR products obtained in 50-μl reactions.
The primers used for Sodalis amplified a 650-bp fragment of the hemolysin gene (accession no. AP008232) were HemF (ATGGGAAACAAACCATTAGCCA) and HemR (TCAAGTGACAAACAGATAAATC). The presence of Wigglesworthia was detected by amplification of 365-bp fragment of the thic gene (accession no. AB063522) with primers ThiCF (TGAAAACATTTGCAAAATTTG) and ThiCR (GGTGTTACATAGCATAACAT). For DNA quality control, the G. morsitans subsp. morsitans tubulin gene (accession no. DQ377071) was amplified with primers GmmTubF (TAGTTCTCTTCAACTTCAGCCTCTT) and GmmTubR (TCGTTGACCATGTCTGGTGT). The presence of Wolbachia was detected by the amplification of a 610-bp fragment of the wsp gene with primers 81F (TGGTCCAATAAGTGATGAAGAAAC) and 691R (AAAAATTAAACGCTACTCCA) for 30 cycles at an annealing temperature of 59°C.
Bacterium-specific PCR amplification conditions consisted of 2 min of initial denaturation at 94°C, followed by 30 cycles of 94°C for 30 s, 54°C for 40 s, and 72°C for 1 min with a final elongation at 72°C for 7 min with a Bio-Rad DYAD Peltier thermocycler. For gmmtub amplification, an annealing temperature of 60°C was used. The amplification products were analyzed by agarose gel electrophoresis and quantified with Kodak 1D image analysis software.
(iii) Real-time quantitative PCR (qPCR).
Sodalis and Wigglesworthia densities relative to the host gene copy number were also determined by qPCR amplification with the iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). The data were analyzed with software version 3.1. For Sodalis, the primers used the amplified chitinase gene (ChitF [TGGGGACAGTACGATGGCAGAGC] and ChitR [GACCATGACGTGGATCACAG]), and the amplification conditions were 40 cycles at 60°C. Wigglesworthia quantification was done by amplifying the thiamine locus with primers ThiC-F (CATGCATGCTATTCGACGTT) and ThiC-R (TTCCGCTAAGCGATCAATTT) at 54°C. Symbiont density is defined as the number of symbiont genomes relative to the host tubulin copy number determined by amplification with primers GmmTubF (CCATTCCCACGTCTTCACTT) and GmmTubR (GACCATGACGTGGATCACAG) for 35 cycles at 60°C as described previously (29). All assays were carried out in duplicate, and three replicates were averaged for each sample. Negative controls were included in all amplification reaction mixtures.
Histology and FISH analysis.
Dissected bacteriomes and milk gland tissues were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-μm-thick sections, and mounted on poly-l-lysine-coated microscopy slides. After dewaxing in methylcyclohexane and rehydration, milk gland sections were processed by the FISH protocol described by Anselme et al. (4). Slides were covered with a drop of 70% acetic acid and incubated at 45°C until the drop dried, followed by dehydration and a 10-min deproteinization step in 0.01 N HCl-pepsin at 37°C. Slides were then dehydrated again, prehybridized for 30 min at 45°C, and hybridized for 3 h at 45°C with 5′-end rhodamine-labeled Sodalis (5′-ACGAGACTCTAGCCT GCCAG-3′)- and Wigglesworthia (5′-ACGATACTCTAGTTTATTAG-3′)-specific 16S rRNA probes, respectively. The slides were then washed and mounted with 4′,6′-diamidino-2-phenylindole (DAPI)-containing Gel Mount (Electron Microscopy Science). For histological analysis of bacteriomes, the slides were dewaxed, rehydrated, stained for 20 min with 1:20-diluted 0.4% Giemsa staining solution (Accustain; Sigma), washed briefly in water, dehydrated, and subsequently mounted in Permount (Fisher Scientific). Microscopic analyses were conducted with a Zeiss Axioskop2 microscope equipped with an Infinity1 USB 2.0 camera and software (Lumenera Corporation). Fluorescent images were taken with a fluorescence filter set with fluorescein-, rhodamine-, and DAPI-specific channels.
Fitness parameters of adult progeny produced by per os ampicillin-treated females. (i) Fecundity.
Nine 10-day-old male progeny produced by per os ampicillin-treated females were divided into three groups. Each group of three males was introduced and allowed to mate with eight 3-day-old wild-type females. The fertility status of the females was monitored visually, and at 30 days postmating, all nonpregnant females were dissected to confirm copulation by the presence of sperm in their spermathecae. Similarly, 3- to 4-day-old female progeny produced by per os ampicillin-treated females were allowed to mate with 1-week-old wild-type males. The fertility status of the females was similarly monitored. The physiological barriers to fecundity were microscopically observed following dissection of reproductive tissues from fertilized female progeny of ampicillin-treated females.
(ii) Hemoglobin levels.
Progeny resulting from per os ampicillin-treated females and their age-matched normal controls were monitored for undigested hemoglobin levels in their gut every 6 h following a blood meal administered as 6-day-old adults. Hemoglobin levels were determined by the cyanmethemoglobin method (catalog no. D5941; Sigma) and expressed as milligrams per gut. For each designated time point, three midguts were used except for 24- and 48-h samples, where six and five samples were used, respectively.
(iii) Longevity of progeny.
The longevity of adult progeny of ampicillin-treated females was compared for 60 days with that of progeny of normal flies. Mortality was recorded, and survival was expressed as a percentage. Three replicate experiments were performed starting with 26, 24, and 20 flies in the control group and 24, 25, and 26 flies in the corresponding experimental group.
(iv) Heat tolerance.
Two groups of flies, adult progeny of ampicillin-treated females and adult progeny of age-matched wild-type control flies, were maintained at 28°C and 55% humidity under the normal blood-feeding regimen. Their mortality was monitored for 60 days as a function of high-temperature exposure.
Trypanosome infection prevalence.
A group of fertile female flies (n = 50) received 50 μg/ml ampicillin at each blood meal, and their third, fourth, and fifth larval depositions were collected and allowed to hatch. The adults received one infectious blood meal containing bloodstream form Trypanosoma brucei subsp. rhodesiense YTat1.1 (1 × 106 trypanosomes/ml blood) in either their first blood meal (48 h posteclosion) or their fourth blood meal. In each experiment, a group of normal, age-matched progeny that received the same infectious blood meal was included as a control. Only flies that took a full blood meal were included in the experiments and were subsequently maintained on a regular blood meal diet for 15 days. The midguts were microscopically dissected, and the presence of the parasite was confirmed. The dissected parasite-infected gut tissue was gently homogenized in cell wash buffer (20 mM Tris HCl, 10 mM NaCl, 3 mM MgCl2), and three independent measurements were done. Where indicated, parasites in each homogenized gut sample were counted with a hemocytometer.
RESULTS
Differential impact of antibiotic treatment on host fecundity.
We evaluated the effects of continuous per os treatment of fertile females with the penicillin-based antibiotics ampicillin and carbenicillin and with tetracycline or rifampin by measuring the total number of larvae deposited per group over the course of three deposition cycles (Table 1). To determine if the penicillin antibiotics have a prolonged effect on the process of metamorphosis within the larvae or pupae, the hatching rate of progeny was determined in these groups (Table 2). A two-way analysis of variance was performed to determine the groups whose fecundity was significantly affected. In general, ampicillin, given either as an injection (once or twice) or via continuous blood meal supplementation (30 or 50 μg/ml), did not have a significant effect on fecundity (P < 0.05). However, in the rifampin (20 μg/ml) and tetracycline (25 μg/ml) groups, there was significant lowering of fecundity (P < 0.05). The puparia obtained in these groups were restricted mostly to the first deposition cycle and did not hatch to give viable adult progeny. In contrast, in general, the hatching rate in the ampicillin groups was similar to that in the control groups. The resulting F1 progeny were mated for fertility effects, but all were sterile, with the exception of the carbenicillin injection groups. Only in the carbenicillin group were we able to look at the effects of the F2 progeny, which was also found to be fertile. Our results with different antibiotic treatments indicate differential fecundity outcomes in that penicillin treatment led to viable but sterile offspring while tetracycline treatment induced sterility in treated females.
TABLE 2.
Effects of antibiotic treatments on pupal hatching rates
| Treatment | Relative pupal hatching rate (%) |
|---|---|
| Control | 100 |
| Ampicillin per os (10 μg/ml) | 78 |
| Carbenicillin per os (10 μg/ml) | 86 |
| 1 ampicillin injection (1 μg/mg body wt) | 93 |
| 2 ampicillin injections (total of 1 μg/mg body wt per injection) | 100 |
| 1 carbenicillin injection (1 μg/mg body wt) | 94 |
| 2 carbenicillin injections (total of 1 μg/mg body wt per injection) | 100 |
Antibiotic treatment effects on symbiont viability.
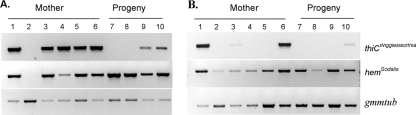
A symbiont species-specific diagnostic PCR assay was developed to monitor the symbiont infection status in the antibiotic-treated fertile females and in their 10- to 13-day-old progeny. Fertile females were dissected into gut and abdomen (devoid of gut and thorax) fractions. The gut material included the bacteriome and midgut tissues, while the abdomen included the reproductive organs and accessory milk gland and the associated extensive tubular duct structures. The presence of the two symbionts (Sodalis, Wigglesworthia) was subsequently determined from each DNA fraction. Sodalis was detected in the midgut and abdomen samples of the ampicillin-treated mothers (lanes 3 to 6) and in their progeny (lanes 7 to 10), as shown in Fig. 1A and B, respectively. Although Wigglesworthia could still be detected in the guts of ampicillin-treated flies (lanes 2 to 6), it was significantly reduced in the milk gland (abdomen) samples. When the progeny from the second gonotrophic cycle were analyzed, ampicillin-receiving groups (continuous and discontinuous injections) did not harbor any Wigglesworthia bacteria that were detectable by the PCR assay (lanes 3 and 4 for the midgut and lanes 7 and 8 for the abdomen, respectively). Neither feeding nor injection of carbenicillin cleared Wigglesworthia infections in the guts of the mothers, and in many cases their milk glands still harbored the bacterium (Fig. 1A, lanes 5 and 6 and 9 and 10, respectively). This finding is also supported by our earlier fertility study, in which carbenicillin-treated females were found to be fecund and gave rise to fertile progeny. In contrast to ampicillin, tetracycline feeding caused a nearly complete clearance of Sodalis and Wigglesworthia in the midgut (Fig. 1A, lane 2) and these females only produced few progeny in their first gonotrophic cycle which did not hatch into adults.
FIG. 1.
PCR assay demonstrating the status of Wigglesworthia and Sodalis infections in gut (A) and abdomen (B) tissues from antibiotic-treated mothers (lanes 1 to 6) and from their adult progeny (lanes 7 to 10). Lanes: 1, control; 2, tetracycline fed; 3, ampicillin injected; 4, ampicillin fed; 5, carbenicillin fed; 6, carbenicillin-injected females; 7, progeny of ampicillin-injected females; 8, progeny of ampicillin-fed females; 9, progeny of carbenicillin-fed females; 10, progeny of carbenicillin-injected females. DNA samples were PCR amplified with diagnostic primers for the presence of Wigglesworthia and Sodalis and for input DNA quality. Negative samples were subjected to a second PCR amplification to confirm the absence of symbionts. This assay was carried out for a minimum of 12 individuals from each group, and one representative analysis is shown here.
Sodalis and Wigglesworthia densities were determined by qPCR in the 10- to 13-day-old adult progeny resulting from the second and third gonotrophic cycles (G2 and G3) of females maintained on ampicillin-supplemented blood meals. No Wigglesworthia bacteria could be detected (data not shown), while Sodalis densities were not significantly different from those of the wild-type control (Fig. 2) (P > 0.05).
FIG. 2.
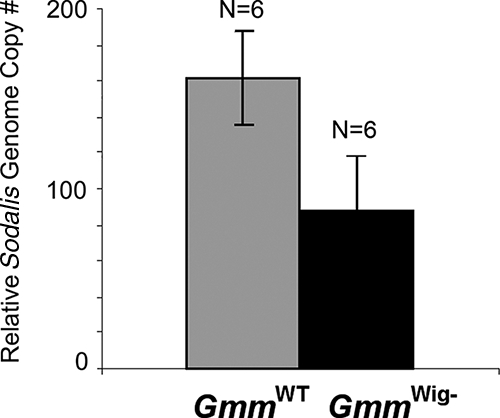
qPCR analysis of Sodalis density in wild-type and GmmWig− females (P value, <0.001). Values were normalized by tsetse tubulin gene copy number.
Microscopic analysis of bacteriome and milk gland organs.
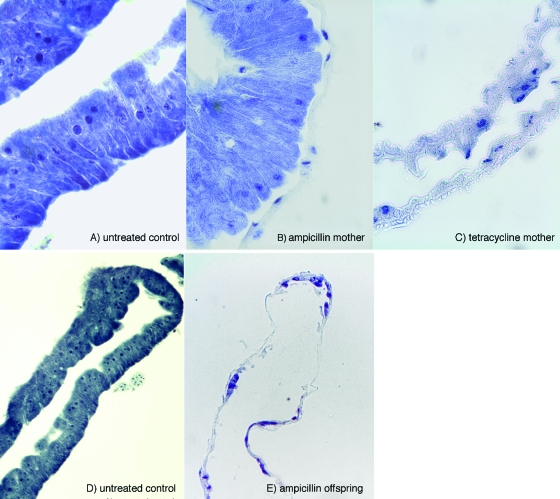
The presence of both Wigglesworthia and Sodalis bacteria was previously shown in the milk gland lumen and also in the case of the latter within the milk gland cells (5). Our PCR analysis could detect the presence of Wigglesworthia in the gut (bacteriome) tissue of both the control and ampicillin-treated flies but not in the abdomens of ampicillin-treated flies. The PCR data were confirmed by histological analysis. Giemsa stain was used to determine the integrity of the bacteriome structure and the presence of intracellular Wigglesworthia. The bacteriomes of 30-day-old per os ampicillin-treated flies showed abundant rod-shaped Wigglesworthia cells in bacteriocytes similar to those observed in untreated control bacteriomes (Fig. 3A). In contrast, tetracycline-treated flies with reduced fecundity showed degenerated bacteriomes with completely empty bacteriocytes. When the bacteriomes from the progeny deposited by ampicillin-treated females were similarly analyzed, Wigglesworthia cells could not be observed (Fig. 3B). In addition, staining of milk gland sections with the DNA stain DAPI showed rodlike bacteria in the lumen, which were clearly absent in the milk glands of ampicillin-treated females (Fig. 3C).
FIG. 3.
Images of bacteriome sections stained with Giemsa at ×40 magnification (A to C) and taken at ×10 magnification (D and E).
FISH analysis of milk gland tissue.
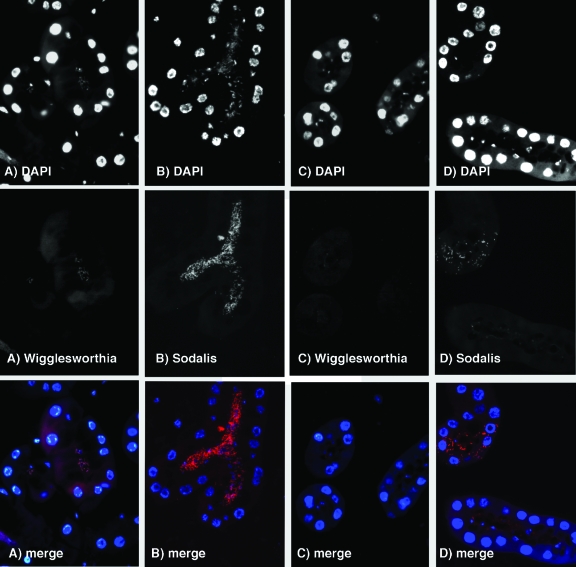
With bacterium-specific 16S rRNA oligonucleotide probes, we were able to identify the rod-shaped bacteria in the milk gland lumen as the symbionts Wigglesworthia and Sodalis, as shown before (5). Interestingly, Sodalis was also localized intracellularly within the cytoplasm of some of the milk gland cells. The numbers of intracellular Sodalis bacteria, however, were significantly lower than those of the extracellular Sodalis bacteria in the lumen. As expected, we could not detect Wigglesworthia in the milk gland lumen of ampicillin-treated mothers, supporting the PCR data, which showed that ampicillin treatment selectively clears the extracellular Wigglesworthia population (Fig. 4). In parallel, the Sodalis population is also cleared from the milk gland lumen of ampicillin-treated flies. However, the intracellular Sodalis bacteria are apparently unaffected by the ampicillin treatment and can still be detectable within the milk gland cells in numbers similar to those observed in normal milk gland tissue. Our results based on PCR and microscopy analyses indicate the presence of two different populations of symbionts in tsetse flies. Wigglesworthia is present within the bacteriocytes in the bacteriome organ in the midgut but also exists as free-living cells in the milk gland lumen. Both intracellular and extracellular infections are noted for Sodalis in the milk gland lumen. Furthermore, mother's milk gland secretions represent the mode of transmission of Wigglesworthia to progeny. When Wigglesworthia bacteria are cleared from the milk gland lumen following ampicillin treatment, the resulting progeny do not harbor Wigglesworthia.
FIG. 4.
Panels A and B show images of cross sections of milk gland tubuli from untreated flies hybridized with rhodamine-labeled Wigglesworthia (A)- and Sodalis (B)-specific probes. Panels C and D show images of cross sections of milk gland tubuli from ampicillin-treated flies hybridized with Wigglesworthia (C)- and Sodalis (D)-specific probes. The top series of images shows the DAPI signal for all DNA; visible are the large nuclei of the milk gland cells along with the bacteria present in the lumen in normal flies but not in antibiotic-treated females. The middle series of images shows the rhodamine signal of the probes, and the bottom series displays the merged images of both DAPI and rhodamine data in color.
Effects of Wigglesworthia on host fitness parameters.
Ampicillin treatment did not affect the viability of the intracellular Wigglesworthia in the bacteriomes or the fecundity of the fertile females but resulted in the deposition of progeny devoid of Wigglesworthia (hereafter denoted as female and male). We further investigated the cost of loss of Wigglesworthia in terms of host longevity, blood meal digestion, and vector competence in GmmWig− progeny.
Reproductive defects.
As already described, female GmmWig− flies were found to be sterile while GmmWig− males were fertile. Our attempts to rescue sterility by provisioning the GmmWig− female diet with a cocktail of vitamin supplementation did not produce any progeny (data not shown). Carbenicillin feeding results mimicked the ampicillin results (Fig. 1 and Table 1). However, the injected carbenicillin was not able to clear either Sodalis or Wigglesworthia from the progeny and they were fertile like the wild-type females (Table 1). The G. morsitans subsp. morsitans flies harbor a third symbiont, Wolbachia, which in other insects can result in various reproductive manipulations. Its functional role in tsetse is unknown, but to rule out possible Wolbachia-mediated effects, we tested our progeny for the presence of Wolbachia with a bacterium-specific PCR amplification assay similar to what we described for Sodalis and Wigglesworthia. We found Wolbachia in both male and female GmmWig− flies, indicating that ampicillin treatment does not have an impact on the Wolbachia bacterium, as expected given its intracellular biology (data not shown). Hence, the reproductive sterility we observed could not be due to Wolbachia-mediated effects. Our results suggest that the intracellular populations of Wigglesworthia bacteria present in the bacteriomes are essential for the development of viable offspring. The exact mechanism of loss of fecundity during the oogenesis, embryogenesis, or larvagenesis process and what is causing the defects in the reproductive cycle in GmmWig− remain unknown and require further study.
In the case of tetracycline-treated flies, it has been noted that vitamin supplementation of experimental females could support the deposition of one or two progeny (26). These puparia, however, rarely hatched, were unfit as adults, and did not live past several days (data not shown). Tetracycline treatment eliminates Wolbachia in addition to Wigglesworthia and Sodalis from treatment flies. To rule out possible Wolbachia-mediated effects, the experimental GmmWig− females were also mated with adult GmmWT males that had been maintained on tetracycline for at least a 2-week period but no difference was noted in their fecundity (data not shown).
Longevity and heat stress.
To determine the potential effect of Wigglesworthia on host longevity, female GmmWig− fly mortality was monitored over the course of 60 days and compared to that of GmmWT flies (Fig. 5A). A slight but significant reduction (P < 0.006) in survival was observed in the treatment group compared to control flies (one-way analysis of variance). When GmmWT and GmmWig− females were maintained at 27°C instead of the 24°C at which the normal colony was maintained, there was a significant difference in the mortality rate, with the Wigglesworthia-deficient flies showing a higher mortality rate (Fig. 5B). The absence of Wigglesworthia impacted both the longevity and the temperature sensitivity of flies.
FIG. 5.
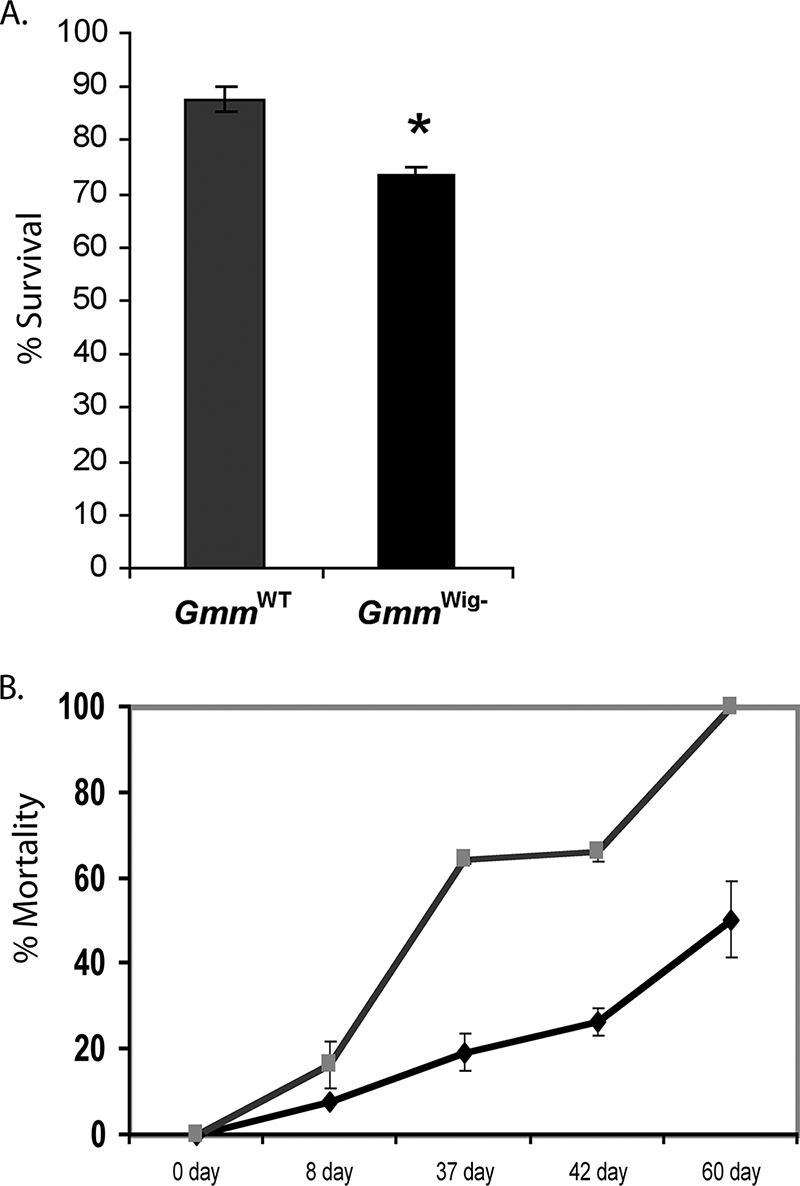
Host survival-associated fitness cost after elimination of Wigglesworthia. (A) Longevity. Percent survival determined over a 60-day period is shown. *, P value of <0.03. (B) Heat tolerance response. Percent survival determined over a 60-day period is shown. ♦ denotes GmmWT females, and indicates GmmWig− females.
Digestion.
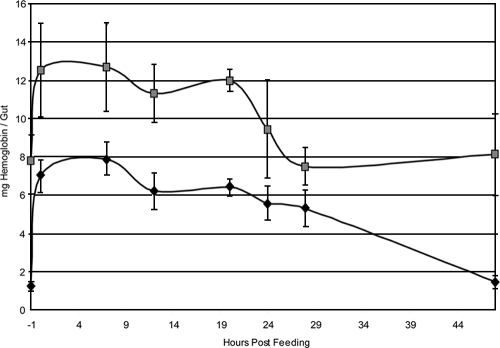
Visual inspection indicated incomplete blood meal digestion in both sexes of GmmWig− flies in comparison to the control GmmWT flies. Hemoglobin levels were used as a measure of blood meal digestion over a time course period following feeding. The hemoglobin levels measured in normal flies were compared to the levels in female GmmWig− flies. The gut hemoglobin levels of the Wigglesworthia-free progeny were higher in the prefed state than those of their normal counterparts. Similarly, following feeding, digestion of hemoglobin was significantly compromised in female GmmWig− flies compared to the control females. At 48 h postfeeding, almost all of the hemoglobin was cleared in the control guts whereas most of the hemoglobin remained undigested in female GmmWig− flies (Fig. 6). These results suggest an important role for Wigglesworthia in the host blood meal digestion process.
FIG. 6.
Time course of hemoglobin digestion in GmmWT and GmmWig− females. Data represent three replicate experiments. The first time point in the gut hemoglobin level analysis corresponds to 1 h before the flies were given a blood meal. This time point represents 48 h after their last blood meal. The bars denote the standard deviations of the multiple samples tested for each time point in the experimental and control groups. denotes GmmWig− females, and ♦ indicates wild-type females.
Vector competence.
To evaluate the potential role of Wigglesworthia in host vector competence, the trypanosome infection process, we compared the parasite midgut infection prevalence in female and male GmmWig− groups with that in normal control flies. It has been observed that acquisition of parasites in the first blood meal of newly emerged (teneral) flies results in higher infection prevalences than when parasites are provided to older previously blood-fed (nonteneral) flies (37). We tested the parasite competence of teneral and nonteneral GmmWig− flies by administering a single infectious blood meal as either the first or the third blood meal of each group, respectively. Each experimental group had a GmmWT control group exposed to the same infectious blood meal. Fifteen days after infection acquisition, midguts were dissected and scored microscopically for the presence of parasites (Table 3). No significant difference was noted in midgut infection prevalence between GmmWig− flies and controls when parasites were provided in the first blood meal (P = 0.27). There was also no significant difference in the number of parasites harbored in each midgut. There was, however, a significant difference in the infection prevalence between normal and GmmWig− flies when infections were initiated in nonteneral adult flies. Infections in the control group were significantly lower than those observed in GmmWig− flies (P < 0.001). The parasite infection density in the GmmWig− flies did not show any variation from that in the control flies (data not shown). Our results indicate that absence of Wigglesworthia has a significant impact on flies' vectorial capacity, resulting in higher parasite infections.
TABLE 3.
Trypanosome infection prevalence in teneral and nonteneral adultsa
| Treatment and replicate group | No. of flies | Infection prevalence (%) | Avg density (no. of parasites/gut) | P value |
|---|---|---|---|---|
| Control (teneral) | ||||
| 1 | 13 | 15 | 6 × 105 | 0.27 |
| 2 | 13 | 0 | 0.27 | |
| 3 | 8 | 25 | 1.2 × 106 | 0.27 |
| Adult GmmWig− (teneral) | ||||
| 1 | 13 | 8 | 2 × 106 | 0.27 |
| 2 | 12 | 41 | 2 × 105 | 0.27 |
| 3 | 7 | 14 | 1 × 105 | 0.27 |
| Control (nonteneral) | ||||
| 1 | 23 | 4 | NDb | <0.001 |
| 2 | 18 | 11 | ND | <0.001 |
| Adult GmmWig− (nonteneral) | ||||
| 1 | 17 | 71 | ND | <0.001 |
| 2 | 13 | 46 | ND | <0.001 |
Teneral flies received parasites in their first blood meal; nonteneral flies received parasites in their fourth blood meal.
ND, not done.
DISCUSSION
Although symbiosis with gut microbiota exists in all metazoans, host factors that maintain this homeostatic relationship remain largely unknown. The nature of the host-symbiont molecular interactions that have led to extreme dependence in insects on a group of bacteriome-associated endosymbionts remains largely unknown. This is the first direct study providing knowledge about the functional biology of the symbiosis of tsetse with the obligate mutualist Wigglesworthia. Our findings provide evidence for a highly integrated role for Wigglesworthia influencing the tsetse fly's important physiological processes, ranging from digestion to reproduction to immunity.
Our results show that the penicillin antibiotics do not affect the intracellular forms of the Wigglesworthia and Sodalis symbionts. The presence of intact Wigglesworthia in the bacteriome organ of antibiotic-treated females enables the maintenance of host fertility and results in a fecundity index (number of puparia deposited by fertile females) comparable to that of control flies. In contrast, antibiotics that eliminate the intracellular forms of Wigglesworthia in the bacteriome compromise host fecundity severely. Supplementation of the host diet with a vitamin cocktail only partially rescues this sterility during tetracycline administration, indicating that Wigglesworthia provisioning of the host diet is complex. The population of Wigglesworthia in the milk gland lumen of fertile females is, however, cleared by ampicillin treatment of the mothers. Furthermore, the progeny of such treated females are devoid of Wigglesworthia, confirming that the milk gland secretions provide the definitive route of transmission of this symbiont to intrauterine larvae. Given the drastically reduced proteome of Wigglesworthia, we had predicted that its survival would necessitate its complementation of various physiological processes by host cell products. Our previous attempts to establish a cell-free system for Wigglesworthia similar to that of Sodalis has failed to date (S. Aksoy, unpublished data; 6). Thus, it is interesting that free-living Wigglesworthia bacteria are detected in the mother's milk. The physical proximity of the Wigglesworthia cells to the large milk gland cell secretory vacuoles is suggestive of their nutritional reliance on host metabolites (G. M. Attardo, unpublished data). It also remains to be determined how these infections in the milk gland organ originate since no intracellular infections are noted in milk gland cells. It is possible that during larval development, the organ becomes infected with Wigglesworthia and continues to maintain these extracellular infections into adulthood.
Free-living enterics and intracellular parasites (such as Rickettsia bacteria) rely on their complex and flexible surface structures as protection from host defense mechanisms and environmental changes. Wigglesworthia bacteria within bacteriocytes lie free in the cytoplasm of host cells, unlike Buchera, which has also had a prolonged intracellular life in its host-provisioned niche but which is surrounded by multiple host membranes. Furthermore, the Wigglesworthia genome encodes enzymes involved in the biosynthesis of lipopolysaccharide and peptidoglycan, which are products integral to the gram-negative cell wall structure (1). In contrast, the obligate Buchnera has restricted membrane biogenesis capability and lacks the ability to synthesize lipopolysaccharides and outer membrane proteins (33). The retention of membrane capabilities by Wigglesworthia may enable protection from the host milk gland environment and defenses while in transit to the intrauterine progeny in mother's milk.
In addition to a robust membrane structure, Wigglesworthia has retained the machinery for the synthesis of a complete flagellar apparatus, including the basal body, hook, filament, and filament cap regions, and the integral membrane proteins required for motility functions, motA and motB. While retention of genes associated with the flagellar operons is suggestive of a functional role, neither a flagellum nor motility has been observed in adult Wigglesworthia bacteriocytes. It is possible that the expression of a functional flagellum during the extracellular life stage in the milk gland might facilitate the transmission of Wigglesworthia cells to the intrauterine progeny via milk secretion. Our ongoing studies of the spatial and temporal flagellar gene expression profile will further elucidate this observation.
Interestingly, the penicillin antibiotics do not impair the transmission of Sodalis to progeny. Although the resident Sodalis population in the milk is apparently cleared, intracellular forms remain within the milk gland cells based on FISH analysis. Our symbiont density analysis in adult GmmWig− flies has indicated Sodalis levels in the midgut comparable to those in GmmWT flies. It is possible that relatively few Sodalis cells transmitted to intrauterine larvae can establish infections and proliferate to reach normal adult density levels during the 1-month-long pupal development period. It remains to be seen if multiple routes of transmission exist for Sodalis, including transovarial and paternal routes. Although the proteome of Sodalis contains most of the Wigglesworthia products, it apparently cannot fulfill the biological role the mutualist symbiont plays in important physiological processes of its tsetse host.
The availability of adult tsetse that are devoid of Wigglesworthia has allowed us to begin to test several host fitness parameters to better understand the role of this symbiosis in tsetse flies. Female GmmWig− flies were completely sterile, and this sterility could not be rescued by vitamin supplementation (data not shown). The male GmmWig− flies were, however, fertile and successfully impregnated females, which deposited progeny with no developmental lag. These results indicate that GmmWig− males harbor fertile sperm and that the spermatogenesis process in the male is unaffected by the absence of Wigglesworthia. While a slight increase in host mortality over the 60-day period was noted, another significant impact we observed was on host digestive physiology. It is quite possible that the longevity cost we observed in GmmWig− flies may be due to inefficient digestion of nutrients. Tsetse flies require a blood meal approximately every 48 h, and flies consume a large volume, which in wild-type flies undergoes diuresis quickly, followed by complete digestion within 48 to 72 h (20). In the case of female GmmWig− flies, blood digestion was significantly inhibited on the basis of both visual observations of flies and measurements of gut hemoglobin content. It remains to be seen how the Wigglesworthia proteome may be contributing directly to host digestive processes through either synthesis of protoheme or glycerophospholipids, fatty acids, and steroids. Alternatively, Wigglesworthia may indirectly affect host gene expression, which may affect digestive processes. In the case of aphids, gene expression in the host bacteriocytes has been shown to be influenced by the presence of Buchnera endosymbionts (41).
Finally, we observed a significant impact on parasite transmission by the older adults. In tsetse, newly emerged or teneral flies are considered the most likely to develop a mature, infective trypanosome infection and flies are rendered less susceptible even after a single blood meal (37). The immature state of the tsetse gut peritrophic matrix, immature host immune responses, and various levels of immune effector peptides synthesized by different tsetse host species are among the suggested factors (3, 25). An additional influential factor is the nutritional status of the fly at the time of the infective blood meal. Nutritional stress in the form of starvation of adult flies can result in substantially greater midgut parasite infection prevalences (19). Other experiments have also incriminated the commensal Sodalis product in enhancing the host vector competence (38). Our results show that newly hatched teneral GmmWig− adults which lack Wigglesworthia but harbor Sodalis show a parasite susceptibility similar to that of control teneral GmmWT flies. It is interesting, however, that older adults, which are typically significantly resistant to parasite infections, are now rendered highly susceptible in GmmWig− in the absence of Wigglesworthia. The role of symbiotic gut fauna in the establishment of host immune responses is increasingly being recognized. In Drosophila, the presence of endosymbiotic fauna has been associated with the activation of the innate immune pathway, which regulates the synthesis of a battery of antimicrobial peptides (31). Activation of the same pathway has been found to render tsetse flies resistant to trypanosome infections (13, 16). Recently, a study of the weevil Sitophilus zeamais, which also harbors an obligate mutualist symbiont, suggested a role for the host peptidoglycan recognition proteins in regulating host-symbiont dynamics (4). Comparative host immune gene expression profiling studies of GmmWT and Wigglesworthia-deficient GmmWig− flies can provide insights into the role of mutualistic symbiosis in the development of host immune physiology and vector competence. The ability to rear tsetse flies that are devoid of Wigglesworthia but harbor Sodalis will now allow us to further elucidate the integrative biology of each of these symbioses independently in the tsetse fly host.
Acknowledgments
This work was generously funded by grants to S.A. from NIGMS (069449), NIAID (051584), the Li Foundation, and the Ambrose Monell Foundation.
We thank Geoffrey Attardo for his help in manuscript preparation and IAEA for tsetse pupana.
Footnotes
Published ahead of print on 8 August 2008.
REFERENCES
- 1.Akman, L., A. Yamashita, H. Watanabe, K. Oshima, T. Shiba, M. Hattori, and S. Aksoy. 2002. Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Genet. 32:402-407. [DOI] [PubMed] [Google Scholar]
- 2.Aksoy, S. 1995. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int. J. Syst. Bacteriol. 45:848-851. [DOI] [PubMed] [Google Scholar]
- 3.Aksoy, S., W. C. Gibson, and M. J. Lehane. 2003. Interactions between tsetse and trypanosomes with implications for the control of trypanosomiasis. Adv. Parasitol. 53:1-83. [DOI] [PubMed] [Google Scholar]
- 4.Anselme, C., A. Vallier, S. Balmand, M. O. Fauvarque, and A. Heddi. 2006. Host PGRP gene expression and bacterial release in endosymbiosis of the weevil Sitophilus zeamais. Appl. Environ. Microbiol. 72:6766-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attardo, G. M., C. Lohs, A. Heddi, U. A. Alam, S. Yildirim, and S. Aksoy. 2008. Analysis of milk gland ultrastructure and function in Glossina morsitans: milk protein production, symbiont populations and fertility. J. Insect Physiol. [Epub ahead of print.] doi: 10.1016/j.jinsphys. 2008.06.008. [DOI] [PMC free article] [PubMed]
- 6.Beard, C. B., S. L. O'Neill, P. Mason, L. Mandelco, C. R. Woese, R. B. Tesh, F. F. Richards, and S. Aksoy. 1993. Genetic transformation and phylogeny of bacterial symbionts from tsetse. Insect Mol. Biol. 1:123-131. [DOI] [PubMed] [Google Scholar]
- 7.Chen, X. A., S. Li, and S. Aksoy. 1999. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J. Mol. Evol. 48:49-58. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, Q., and S. Aksoy. 1999. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol. Biol. 8:125-132. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, Q., T. D. Ruel, W. Zhou, S. K. Moloo, P. Majiwa, S. L. O'Neill, and S. Aksoy. 2000. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med. Vet. Entomol. 14:44-50. [DOI] [PubMed] [Google Scholar]
- 10.Dale, C., and I. Maudlin. 1999. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int. J. Syst. Bacteriol. 49(Pt. 1):267-275. [DOI] [PubMed] [Google Scholar]
- 11.Dale, C., and S. C. Welburn. 2001. The endosymbionts of tsetse flies: manipulating host-parasite interactions. International J. Parasitol. 31:628-631. [DOI] [PubMed] [Google Scholar]
- 12.Dobson, S., K. Bourtzis, H. Braig, B. Jones, W. Zhou, F. Rousset, and S. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 13.Hao, Z., I. Kasumba, M. J. Lehane, W. C. Gibson, J. Kwon, and S. Aksoy. 2001. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc. Natl. Acad. Sci. USA 98:12648-12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann, A. A., and M. Turelli. 1997. Cytoplasmic Incompatibility in insects, p. 42-80. In S. L. O'Neill, A. A. Hoffmann, and J. H. Werren (ed.), Influential passengers. Oxford University Press, Oxford, United Kingdom.
- 15.Holmes, D. S., and J. Bonner. 1973. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry 12:2330-2338. [DOI] [PubMed] [Google Scholar]
- 16.Hu, C., and S. Aksoy. 2006. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol. Microbiol. 60:1194-1204. [DOI] [PubMed] [Google Scholar]
- 17.Jeyaprakash, A., and M. Hoy. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9:393-405. [DOI] [PubMed] [Google Scholar]
- 18.Koga, R., T. Tsuchida, M. Sakurai, and T. Fukatsu. 2007. Selective elimination of aphid endosymbionts: effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol. Ecol. 60:229-239. [DOI] [PubMed] [Google Scholar]
- 19.Kubi, C., J. van den Abbeele, R. De Deken, T. Marcotty, P. Dorny, and P. van den Bossche. 2006. The effect of starvation on the susceptibility of teneral and non-teneral tsetse flies to trypanosome infection. Med. Vet. Entomol. 20:388-392. [DOI] [PubMed] [Google Scholar]
- 20.Langley, P. A. 1977. Physiology of tsetse flies (Glossina spp.) (Diptera: Glossinidae): a review. Bull. Entomol. Res. 67:523-574. [Google Scholar]
- 21.Leak, S. G. A. 1999. Tsetse biology and ecology. CABI, Wallingford, United Kingdom.
- 22.Ma, W. C., and d. L. Denlinger. 1974. Secretory discharge and microflora of milk gland in tsetse flies. Nature 247:301-303. [Google Scholar]
- 23.Moloo, S. K. 1971. An artificial feeding technique for Glossina. Parasitology 63:507-512. [DOI] [PubMed] [Google Scholar]
- 24.Montllor, C. B., A. Maxmen, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189-195. [Google Scholar]
- 25.Nayduch, D., and S. Aksoy. 2007. Refractoriness in tsetse flies (Diptera: Glossinidae) may be a matter of timing. J. Med. Entomol. 44:660-665. [DOI] [PubMed] [Google Scholar]
- 26.Nogge, G. 1981. Significance of symbionts for the maintenance of an optimal nutritional state for successful reproduction in haematophagous arthropods. Parasitology 82:101-104. [Google Scholar]
- 27.Nogge, G. 1976. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experientia 32:995-996. [DOI] [PubMed] [Google Scholar]
- 28.Oliver, K. M., J. A. Russell, N. A. Moran, and M. S. Hunter. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 100:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rio, R. V., Y. N. Wu, G. Filardo, and S. Aksoy. 2006. Dynamics of multiple symbiont density regulation during host development: tsetse fly and its microbial flora. Proc. Biol. Sci. 273:805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell, J. A., and N. A. Moran. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. Biol. Sci. 273:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu, J. H., S. H. Kim, H. Y. Lee, J. Y. Bai, Y. D. Nam, J. W. Bae, D. G. Lee, S. C. Shin, E. M. Ha, and W. J. Lee. 2008. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319:777-782. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai, M., R. Koga, T. Tsuchida, X. Y. Meng, and T. Fukatsu. 2005. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl. Environ. Microbiol. 71:4069-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shigenobu, S., H. Watanabe, M. Hattori, Y. Sakaki, and H. Ishikawa. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81-86. [DOI] [PubMed] [Google Scholar]
- 34.Toh, H., B. L. Weiss, S. A. Perkin, A. Yamashita, K. Oshima, M. Hattori, and S. Aksoy. 2006. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 16:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuchida, T., R. Koga, and T. Fukatsu. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. [DOI] [PubMed] [Google Scholar]
- 36.Welburn, S. C., and I. Maudlin. 1991. Rickettsia-like organisms, puparial temperature and susceptibility to trypanosome infection in Glossina morsitans. Parasitology 102:201-206. [DOI] [PubMed] [Google Scholar]
- 37.Welburn, S. C., and I. Maudlin. 1992. The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Ann. Trop. Med. Parasitol. 86:529-536. [DOI] [PubMed] [Google Scholar]
- 38.Welburn, S. C., and I. Maudlin. 1999. Tsetse-trypanosome interactions: rites of passage. Parasitol. Today 15:399-403. [DOI] [PubMed] [Google Scholar]
- 39.Werren, J. H., D. Windsor, and L. R. Guo. 1995. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 262:197-204. [Google Scholar]
- 40.Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. B Biol. Sci. 261:55-63. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, A. C., H. E. Dunbar, G. K. Davis, W. B. Hunter, D. L. Stern, and N. A. Moran. 2006. A dual-genome microarray for the pea aphid, Acyrthosiphon pisum, and its obligate bacterial symbiont, Buchnera aphidicola. BMC Genomics 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zientz, E., T. Dandekar, and R. Gross. 2004. Metabolic interdependence of obligate intracellular bacteria and their insect hosts. Microbiol. Mol. Biol. Rev. 68:745-770. [DOI] [PMC free article] [PubMed] [Google Scholar]