Abstract
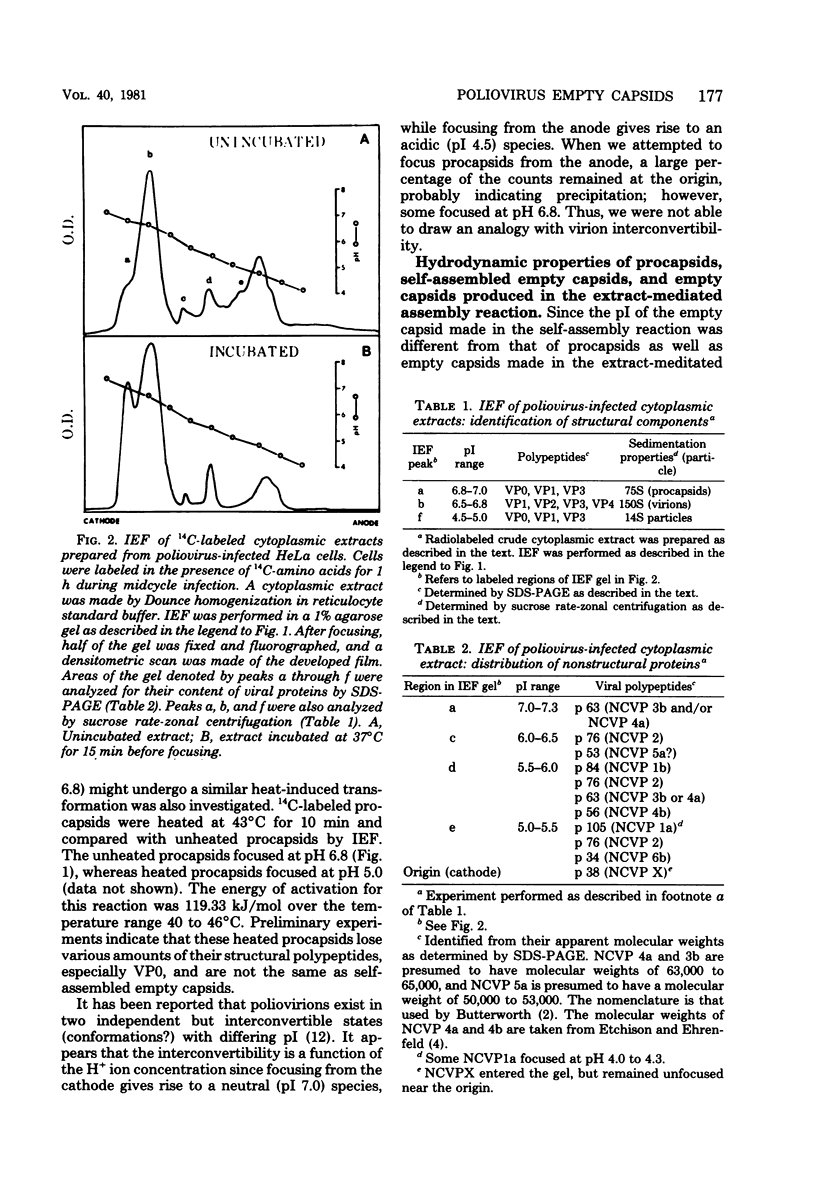
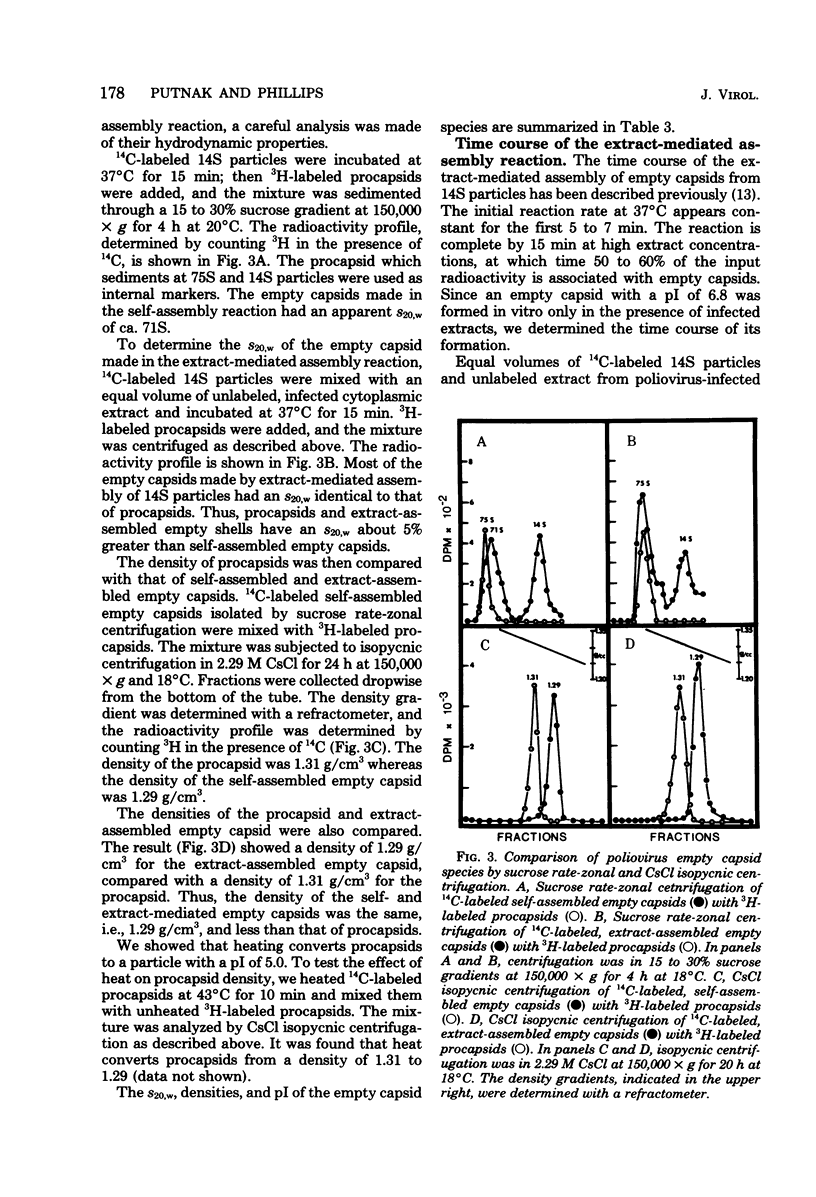
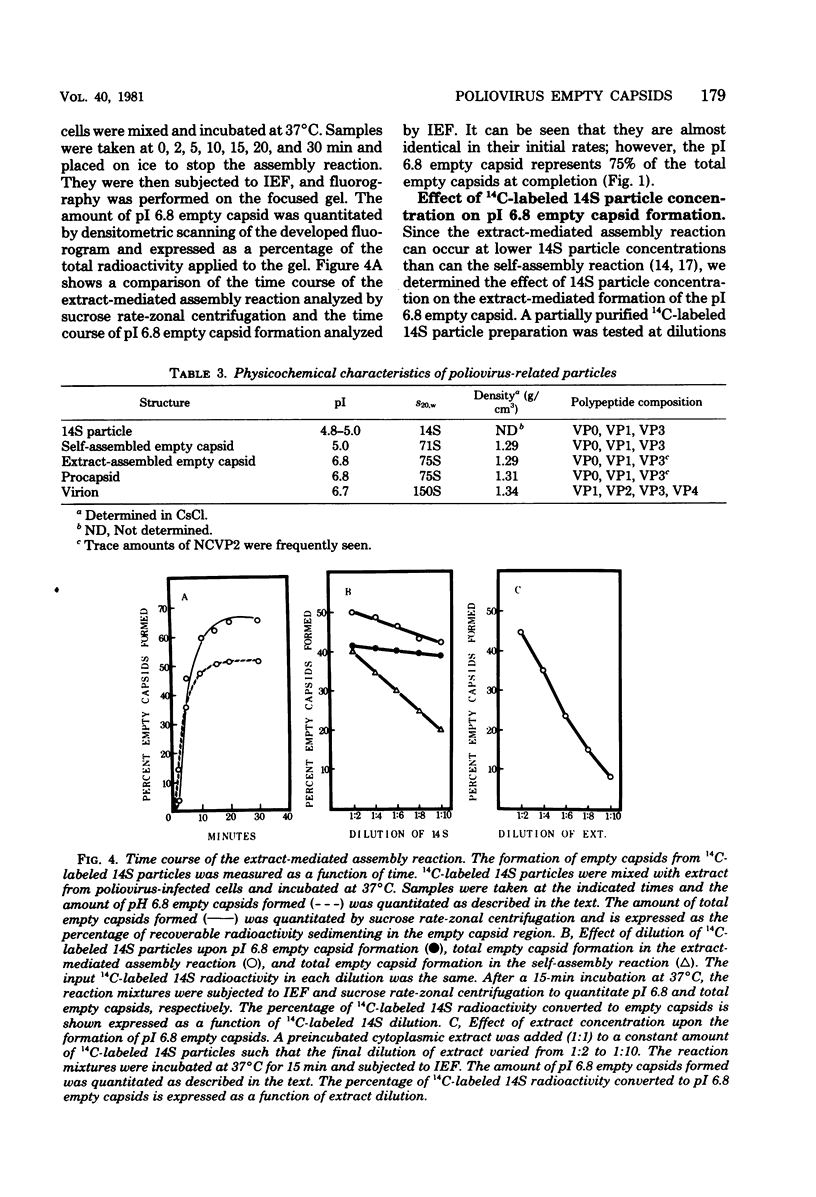
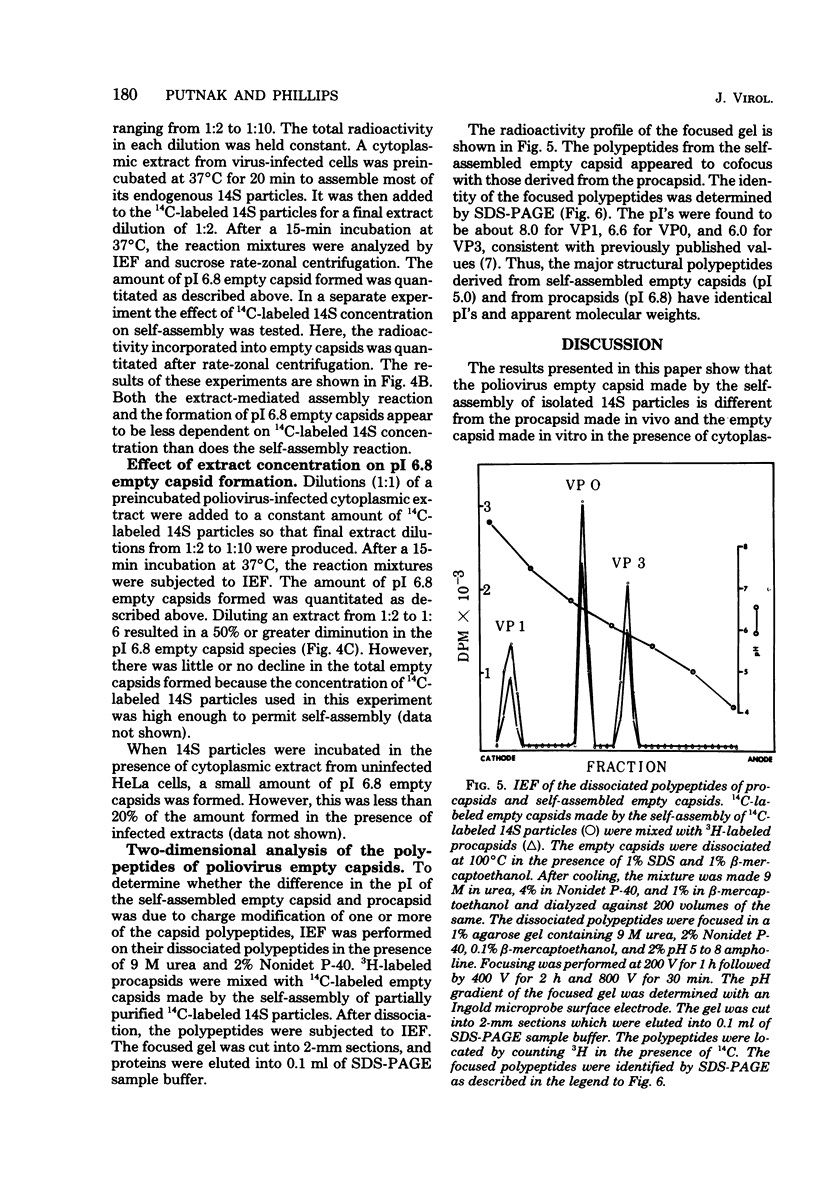
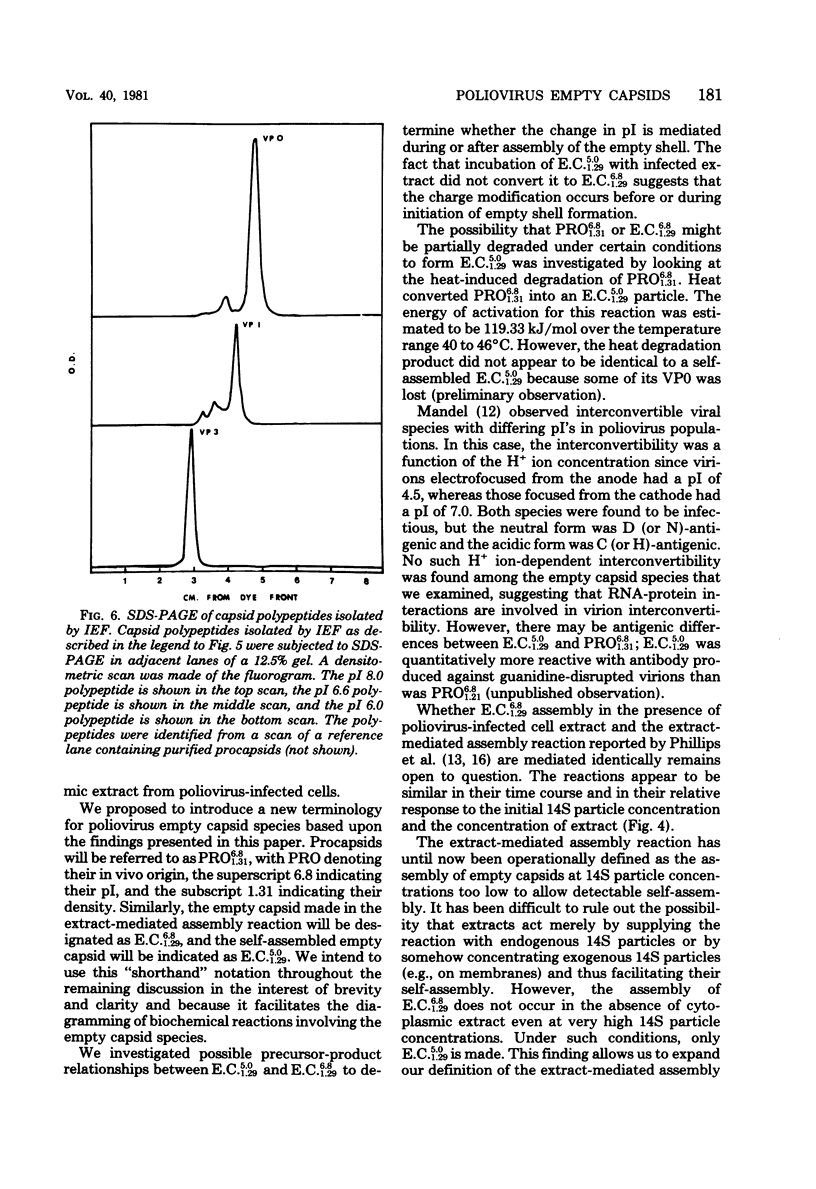
Empty capsid species formed from the self- and extract-mediated assembly of poliovirus type 1 14S particles in vitro and procapsids isolated from virus-infected cells were subjected to isoelectric focusing in charge-free agarose gels. The empty capsid formed in the self-assembly reaction had an isoelectric point (pI) of 5.0, whereas procapsids and extract-assembled empty capsids focused at pH 6.8. Unreacted 14S particles focused at pH 4.8 to 5.0. The sedimentation coefficient (s20,w) and density of the empty capsid species were also determined. Procapsids had a density in CsCl of 1.31 g/cm3, whereas empty capsids formed by self- or extract-mediated assembly had a density of 1.29 g/cm3. Both extract-assembled empty capsids and procapsids had an s20,w of 75S, whereas self-assembled empty capsids had an s20,w of 71S. Self-assembled empty capsids were not converted to pI 6.8 empty capsids by incubation with poliovirus-infected HeLa cell extracts. The dissociated polypeptides of self-assembled empty capsids (pI 5.0) and procapsids (pI 6.8) behaved identically when analyzed by isoelectric focusing in the presence of 9 M urea and by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. These results suggest that infected cell extracts possess a factor that influences the final conformation of the empty shell (pI 6.8, 75S) formed from 14S particles and that this influences is exerted at the initiation step or during the polymerization reaction. A small amount of this activity (less than or equal to 20% of infected extracts) was detected in uninfected cells; the significance of this remains unknown.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghe D. V., Boeyé A. A new species of poliovirus top component. Arch Gesamte Virusforsch. 1973;41(1):138–142. doi: 10.1007/BF01249940. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Carthew P., Martin S. J. The iodination of bovine enterovirus particles. J Gen Virol. 1974 Sep;24(3):525–534. doi: 10.1099/0022-1317-24-3-525. [DOI] [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Viral polypeptides associated with the RNA replication complex in poliovirus-infected cells. Virology. 1980 Nov;107(1):135–142. doi: 10.1016/0042-6822(80)90279-2. [DOI] [PubMed] [Google Scholar]
- Ghendon Y. Z., Yakobson E. A. Antigenic specificity of poliovirus-related particles. J Virol. 1971 Oct;8(4):589–590. doi: 10.1128/jvi.8.4.589-590.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghendon Y., Yakobson E., Mikhejeva A. Study of some stages of poliovirus morphogenesis in MiO cells. J Virol. 1972 Aug;10(2):261–266. doi: 10.1128/jvi.10.2.261-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Reichel C., Wiegers K. J., Drzeniek R. Isoelectric points of polypeptides of standard poliovirus particles of different serological types and of empty capsids and dense particles of poliovirus type 1. J Gen Virol. 1978 Mar;38(3):567–570. doi: 10.1099/0022-1317-38-3-567. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Katagiri S., Aikawa S., Hinuma Y. Stepwise degradation of poliovirus capsid by alkaline treatment. J Gen Virol. 1971 Oct;13(1):101–109. doi: 10.1099/0022-1317-13-1-101. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Yin F. H., Noble-Harvey J. Fractionation of biologically active and inactive populations of human rhinovirus type 2. Virology. 1975 Feb;63(2):384–394. doi: 10.1016/0042-6822(75)90311-6. [DOI] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of poliovirus. II. Evidence for the self-assembly of 14 S particles into empty capsids. Virology. 1971 May;44(2):307–316. doi: 10.1016/0042-6822(71)90262-5. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of polioviruses. I. Kinetics of the assembly of empty capsids and the role of extracts from infected cells. Virology. 1969 Dec;39(4):811–821. doi: 10.1016/0042-6822(69)90018-x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. The morphogenesis of poliovirus. Curr Top Microbiol Immunol. 1972;58:157–174. doi: 10.1007/978-3-642-65357-5_5. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Wiemert S. In vitro assembly of poliovirus. V. Evidence that the self-assembly activity of 14 S particles is independent of extract assembly factor(s) and host proteins. Virology. 1978 Jul 1;88(1):92–104. doi: 10.1016/0042-6822(78)90113-7. [DOI] [PubMed] [Google Scholar]
- Yafal A. G., Palma E. L. Morphogenesis of foot-and-mouth disease virus. I. Role of procapsids as virion Precursors. J Virol. 1979 Jun;30(3):643–649. doi: 10.1128/jvi.30.3.643-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H. Involvement of viral procapsid in the RNA synthesis and maturation of poliovirus. Virology. 1977 Oct 15;82(2):299–307. doi: 10.1016/0042-6822(77)90005-8. [DOI] [PubMed] [Google Scholar]