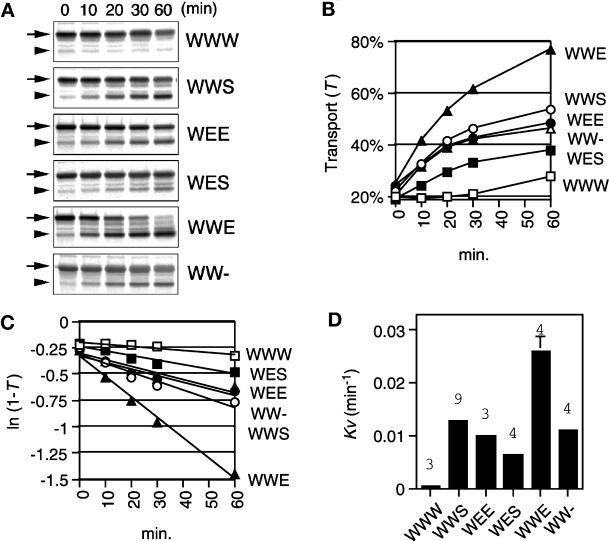
Figure 2.
Transport to the vacuole is accelerated by the presence of the cytoplasmic domain of Emp24p. (A) Autoradiograms of the pulse–chase experiments. Reporter proteins (indicated on the right; see Figure 1) were expressed in yeast cells, pulse labeled for 10 min with [35S]methionine, added with excess methionine, and chased up to 60 min. Cells were lysed at indicated time points during the chase, and the labeled reporter proteins were immunoprecipitated by anti-invertase antiserum. Samples were Endo H treated and analyzed by autoradiography after SDS-PAGE (see MATERIALS AND METHODS for details). Labeled full-length forms (arrows) and processed forms (arrowheads) of the reporter proteins were indicated. (B) Quantitation of the transport to the vacuole. For each sample, the amount of a full-length form and a processed form were densitometrically quantified from an autoradiogram. The degree of transport (T) was calculated as described in MATERIALS AND METHODS and plotted against chase time for each reporter. (C) Logarithmic plots of the degree of remaining substrate for transport (1−T). All the reporters gave linear profiles up to 60 min of chase. (D) Rate constants of transport to the vacuole. The rate constants (kv) were calculated as described in MATERIALS AND METHODS. Bars, mean values; error bars, SD. The numbers of experiments are shown on top of the bars.