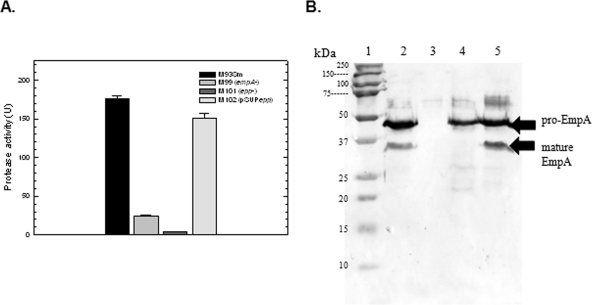
FIG. 3.
(A) Protease activity of V. anguillarum strains M93Sm, M99 (empA mutant), M101 (epp mutant), and M102 (pSUPepp) grown to stationary phase in NSSM. Cultures were grown with shaking for 18 h in NSSM at 27°C (∼2 × 109 CFU/ml). Cells were harvested by centrifugation and the supernatants were filtered through a 0.22-μm filter. Protease activity was determined and normalized to CFU. Samples from left to right: M93Sm, M99, M101, and M102. The data presented are from a representative experiment. Experiments were performed in triplicate and repeated at least three times. Error bars indicate 1 standard deviation. (B) Western blot analysis of EmpA secretion and activation. TCA-precipitated proteins from 1.5 ml of 18-h cell-free culture supernatants (∼80 μg protein) from strains M93Sm, M99 (empA mutant), M101 (epp mutant), and M102 (pSUPepp) grown in NSSM were separated by SDS-PAGE, transferred to nitrocellulose, probed with rabbit anti-LasB antibody followed by immunoglobulin G-labeled goat anti-rabbit antibody, and visualized using TMB as described in Materials and Methods. Lanes: 1, molecular size protein ladder; 2, M93Sm; 3, M99 (empA mutant); 4, M101 (epp mutant); 5, M102 (pSUPepp). The positions of secreted pro-EmpA (∼48 kDa), and mature EmpA (∼36 kDa) are indicated with arrows on the right. The data presented are from a representative experiment. Experiments were repeated at least three times.