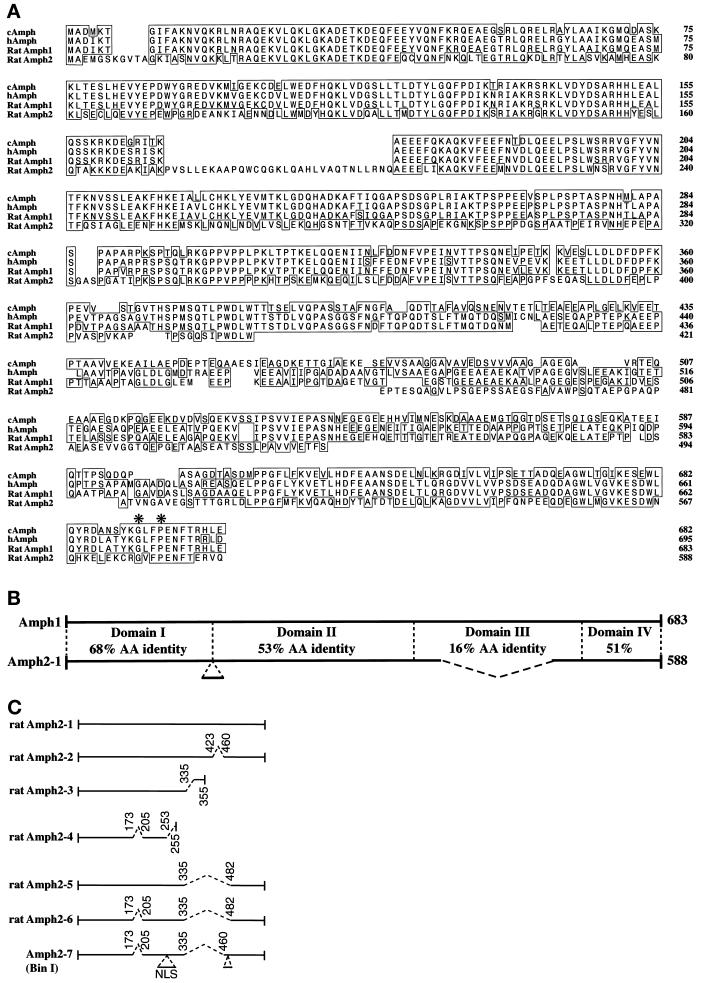
Figure 1.
Primary structure of rat Amph2. (A) Sequence alignment showing homology of rat Amph2 to Amph1. Boxes show regions of amino acid identity in comparison to rat Amph1. The chick and human Amph sequences are shown for comparison. Amino acids conserved in among all SH3 domains are indicated with an asterisk. These sequences have been submitted to the GenBank database under accession numbers Y13380 (Amph2) and Y13381 (Amph1). (B) Overall comparison of Amph1 and Amph2. Amph is divided into four domains. Domain A is predicted to be α-helical and has a average pI of 9.0. Domain B is proline rich and acidic. Domain C is not conserved and acidic. Domain D is an SH3 module and acidic. (C) Amph2 splice variants. Comparison of Amph2 clones. Spliced introns are indicated by dotted lines. Note that Amph2–3 and Amph2–4 contain Amph2 sequence at their 3′ untranslated regions and, therefore, are unlikely to be cloning artifacts.