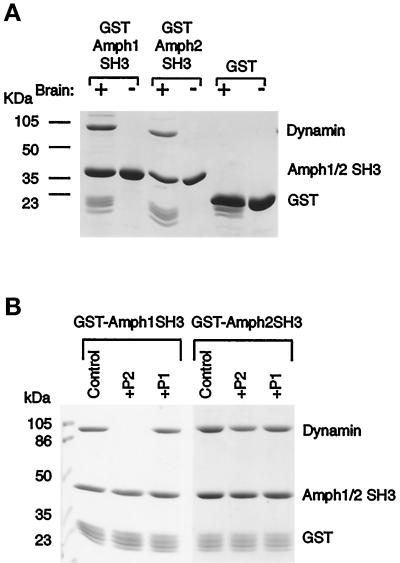
Figure 4.
Amph2 associates with dynamin in vitro. (A) The SH3 domain of Amph2, like Amph1, binds specifically and stoichiometrically to dynamin in brain extracts. GST fusion proteins, and GST as a control, were incubated with brain extract followed by analysis of bound proteins by SDS-PAGE and Coomassie staining. Note the presence of a fainter ∼145-kDa band (probably synaptojanin). (B) The P2 peptide differentially affects dynamin binding to Amph1 and Amph2. GST-tagged SH3 domains were incubated as in A but in the presence or absence of each of the indicated peptides (at a concentration of 200 μM). Bound dynamin was visualized by Coomassie blue staining.