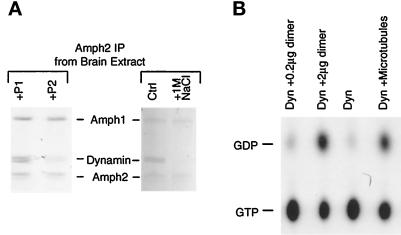
Figure 7.
The heterodimer binds tightly to dynamin and increases its GTPase. (A) Effect of peptides and high salt treatment on dynamin binding to the heterodimer. Amph2 immunoprecipitations of brain extract were done in buffer A containing 200 μM peptide P1 or P2 (left) or in different salt concentrations (right; Ctrl, low salt [buffer A]; 1M NaCl, high salt wash) and separated by SDS-PAGE on 7% gels for Coomassie staining. (B) The heterodimer activates dynamin’s GTPase in vitro. Purified dynamin was incubated with [α-32P]GTP in the presence of different effectors. The heterodimer (0.2 μg in lane 1 and 2.0 μg in lane 2) was purified from brain cytosol by Amph2 immunoprecipitation. All reactions were brought to the same dynamin concentration (0.5 μg in 50 μl). Taxol-polymerized microtubules were included as a positive control.