Figure 9.
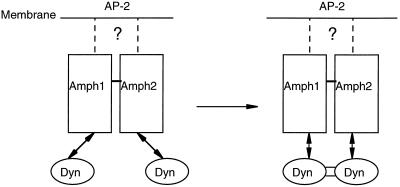
Hypothetical model for the action of the Amph heterodimer in dynamin recruitment. The SH3 domains of both isoforms are accessible in the dimer to interact with separate dynamin molecules, which are brought into close proximity, catalyzing the formation of intermolecular links leading to oligomerization. The heterodimer is likely to be membrane localized through its interaction with AP-2 adaptor complexes (the question mark in the diagram). Amph1 and Amph2 probably dimerize through coiled-coil interactions between their N-terminal α-helical domains (a region weakly conserved with the Rvs proteins). Dissociation of the complex could be brought about by dynamin’s GTPase activity, breaking up the oligomer and returning the separate components to the cytosol.