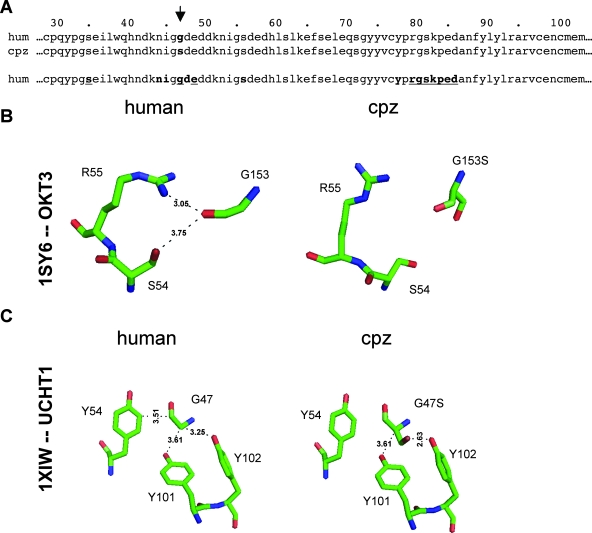
FIG. 3.
Species-specific differences in the UCHT1/CD3ɛ chain complex or OKT3/CD3ɛ chain complex. (A) Amino acid sequence alignment of the human (hum) and chimpanzee (cpz) CD3ɛ chains. The partial sequences represent the sequence that in previously published crystal structures was shown to contribute to human CD3 interaction with the anti-CD3 MAbs OKT3 and UCHT1. The arrow indicates the Gly/Ser-47 change in chimpanzee CD3ɛ (residues are in boldface). In the bottom sequence, CD3ɛ contact residues with UCHT1 are printed in bold, while CD3ɛ contact residues with OKT3 are underlined. To predict structural changes due to the G47S residue mutation for both complexes, we modeled side chain conformational changes on a flexible protein backbone, by using ROSETTA++ to perform optimization of backbone displacement and backbone-dependent side chain rotamer conformations. (B) Predicted conformational changes due to the G47S exchange in the OKT3/CD3ɛ complex. (C) Predicted conformational changes due to the G47S exchange in the UCHT1/CD3ɛ complex.