Abstract
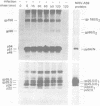
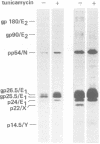
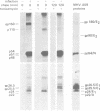
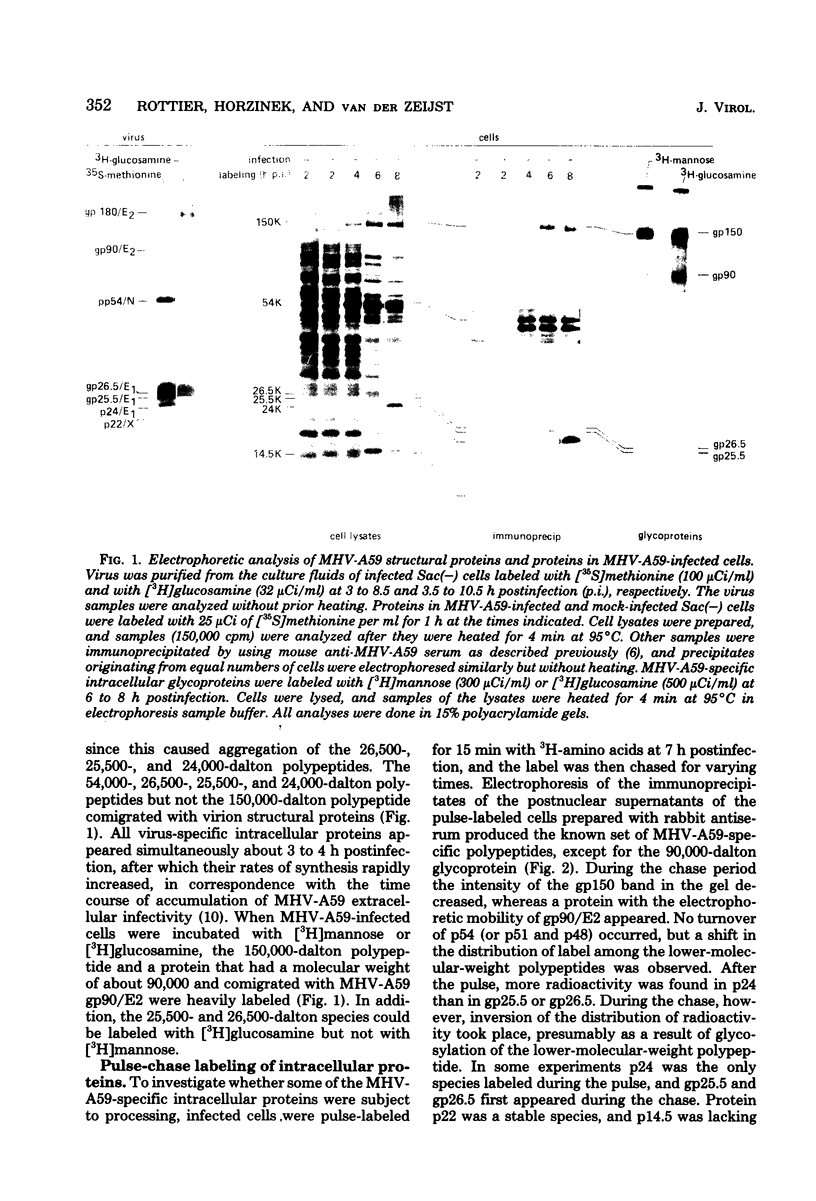
We identified eight protein species in virions of mouse hepatitis virus strain A59. Based on their sizes, prosthetic groups, and locations in virions, these proteins were designated gp180/E2, gp90/E2, pp54/N, gp26.5/E1, gp25.5/E1, p24/E1, p22/X, and p14.5/Y. The positions of the last two proteins in virions are not known. Host protein synthesis in Sac(-) cells infected with mouse hepatitis virus strain A59 was inhibited, and the following novel proteins appeared: gp150, gp90, p54, gp26.5, gp25.5, p24, p22, and p14.5. Except for gp150, these polypeptides all co-electrophoresed with mouse hepatitis virus strain A59 structural proteins. In addition, all of these proteins could be immunoprecipitated with a convalescent mouse serum or a rabbit antiserum raised against purified disrupted virus. After a 15-min pulse of infected cells with radioactive amino acids at 7h postinfection, gp90 was not detected, whereas gp26.5 and gp25.5 were only labeled to a small extent. During a subsequent chase period gp150 was processed to gp90, whereas the radioactivity in gp26.5 and gp25.5 increased concomitantly with a reduction of label in p24. Tunicamycin, an antibiotic which inhibits the synthesis of glycopeptides bearing N glycosidically linked oligosaccharides, prevented the appearance of gp150 in mouse hepatitis virus strain A59-infected cells. Instead, a 110,000-dalton protein accumulated. In contrast, the syntheses of the smaller viral glycoproteins gp26.5 and gp25.5 were resistant to this drug, indicating that these glycosylations were of the O glycosidical type. Although the production of infectious virus in tunicamycin-treated cells was inhibited by more than 99%, release of noninfectious viral particles continued. An analysis of these particles revealed that they lacked the peplomeric glycoproteins gp90/E2 and gp180/E2. Obviously, although the surface projections were not essential for budding of virus particles from the cells, they were required for infectivity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Cheley S., Haworth-Hatherell E. Comparison of polypeptides of two strains of murine hepatitis virus. Virology. 1979 Sep;97(2):492–494. doi: 10.1016/0042-6822(79)90363-5. [DOI] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R. K., Banerjee D. K., Waechter C. J., Olden K., Friedman R. M. Interferon treatment inhibits glycosylation of a viral protein. Nature. 1980 Oct 2;287(5781):454–456. doi: 10.1038/287454a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: intracellular protein synthesis. J Gen Virol. 1981 Mar;53(Pt 1):145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. II. Mapping the avian infectious bronchitis virus intracellular RNA species to the genome. J Virol. 1980 Nov;36(2):440–449. doi: 10.1128/jvi.36.2.440-449.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Lai M. M. Phosphoproteins of murine hepatitis viruses. J Virol. 1979 Nov;32(2):672–675. doi: 10.1128/jvi.32.2.672-675.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]