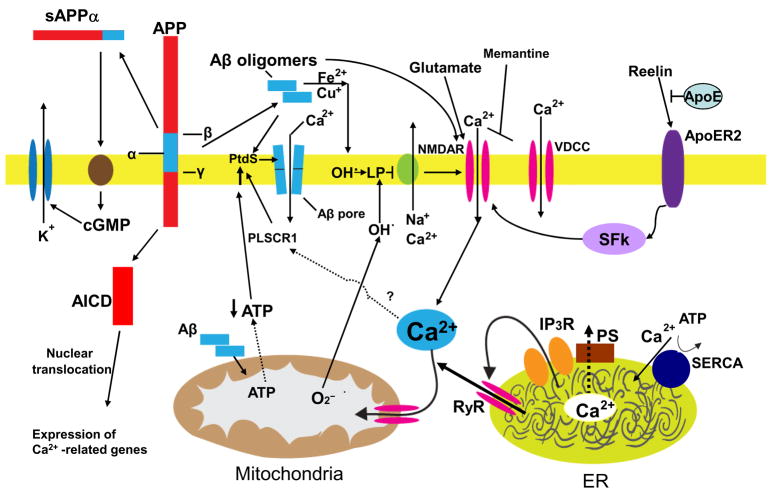
Figure 1.
Mechanisms that may result in perturbed neuronal Ca2+ homeostasis in Alzheimer’s disease. Sequential cleavages of the β-amyloid precursor protein (APP) by β-secretase (β) and γ-secretase (γ) generates the amyloid β-peptide (Aβ). Aβ forms oligomers which can insert into the plasma membrane and form pores through which Ca2+ passes into the cytoplasm. The interaction of Aβ with the plasma membrane may be facilitated by binding to phosphatidylserine (PtdS); age/AD-related mitochondrial impairment (ATP depletion) may trigger flipping of PtdS from the inner portion of the plasma membrane to the cell surface. The PtdS flipping may also result from Ca2+ influx or release from the endoplasmic reticulum (ER) or mitochondria which can activate a phospholipid scramblase (PLSCR1). Aβ can also interact with Fe2+ and Cu+ to generate hydrogen peroxide and hydroxyl radical (OH.) resulting in membrane lipid peroxidation which generates toxic aldehydes that impair the function of membrane ion-motive ATPases (Na+ and Ca2+ pumps). As a result the membrane becomes depolarized and glutamate receptor channels (NMDAR, N-methyl-D-aspartate receptor) and voltage-dependent Ca2+ channels (VDCC) open and flux toxic amounts of Ca2+ into the cytoplasm. In addition, Aβ acts on mitochondria (either directly or indirectly by causing elevated cytoplasmic Ca2+ levels and oxidative stress) to cause increased free radical (superoxide anion radical; O2−) production, Ca2+ overload and decreased ATP production. Amyloidogenic processing also generates an intracellular APP domain (AICD) which can translocate to the nucleus and modify gene transcription in ways that perturb Ca2+ homeostasis. Studies of the presenilins (PS) have implicated ER Ca2+ mishandling in AD. PS functions as an ER Ca2+ leak channel and FAD PS mutations impair this Ca2+ leak channel function resulting in excessive accumulation of Ca2+ in the ER and, as a consequence, enhances Ca2+ release through ryanodine receptor (RyR) and IP3 receptor (IP3R) channels. There is also evidence that PS can interact directly or indirectly with RyR and SERCA (smooth endoplasmic reticulum Ca2+-ATPase) to alter ER Ca2+ release and uptake. Interaction of the protein reelin with the apolipoprotein E receptor (ApoER2) enhances Ca2+ influx through NMDA receptor channels by a mechanism involving a src family tyrosine kinsase (SFk); ApoE can block this effect of reelin. Finally, amyloidogenic APP processing may prevent α-secretase (α) cleavage of APP which would otherwise generate a secreted form of APP (sAPPα). sAPPα is normally produced in response to synaptic activity and is known to activate a signaling pathway involving cyclic guanosine monophosphate (cGMP) that activates K+ channels, thereby hyperpolarizing the membrane and reducing Ca2+ influx. The mechanisms illustrated here include abundant targets for therapeutic intervention (see also Fig. 3) and one drug that targets the NMDA receptor (memantine) is currently used to treat AD patients.