Abstract
The Food and Drug Administration requires rigorous testing of generic formulations of antiepileptic drugs to assure bioequivalence to the brand product and asserts that all approved formulations are interchangeable. Physician surveys, case reports, and “switchback” rates from large-scale generic conversions imply that all generic formulations may not be equal to the brand drug for all patient groups. This review presents the current state of the data on bioequivalence and therapeutic equivalence and proposes a series of studies to better clarify the risks of generic formulation substitution in susceptible populations. Until such studies are completed, when switching to generic formulations, health-care providers and people with epilepsy would do well to proceed cautiously and understand the potential risks and benefits of substitution. Extra caution may be needed for patients at highest risk of seizure complications, such as the pregnant patient, patients with recurrent status epilepticus, or patients who have been seizure-free for long periods of time and are driving.
Generic drugs: the phrase has turned into a flashpoint for physicians, patient groups, the public, and the U.S. Food and Drug Administration (FDA). Widespread generic drug substitution is touted as an important weapon to tame the growth of the multibillion-dollar prescription drug bill borne by the health-care system. In 2002, the FDA estimated that generic drug substitution saved the American public $56.7 billion per year and each 1% increase in generic drug use could save an additional $1.32 billion per year (1). Professional and patient support organizations around the world are concerned about patient safety with indiscriminate generic formulation substitution and have issued statements opposing replacement of prescription drugs without the physician's approval—in particular, stating that generic drug variability can be highly problematic for people with epilepsy (2). In response, articles in the press have implied that the largest support group for people with epilepsy may have compromised its neutrality on this issue by accepting funds from the pharmaceutical industry (3). The FDA states that it has no reliable documentation that generic drugs have ever caused problems for people with epilepsy and that any approved generic drug should be interchangeable with a brand-name product or another generic formulation. What are the bases of these claims and how can the controversy be resolved?
The U.S. Food and Drug Administration's Position on Generic Drugs
The FDA, the governmental body that has responsibility for approving generic formulations of brand-name drugs, has made its position clear. The FDA asserts that the methods used to approve generic formulations are sufficiently rigorous that patients and health-care providers can expect that generic equivalents will provide the same therapeutic effect as brand-name drugs (4). Furthermore, it is the FDA's contention that switches can be made between brand and generic or among generic formulations without concern about loss of therapeutic effect or enhanced toxicity and that no additional testing of patients who have undergone such formulation switches is necessary. These recommendations hold true for all therapeutic categories, whether the compound is used to treat a minor infection or life-threatening illnesses, such as cardiac arrhythmias, immunosuppression for organ transplantation, or seizures. The recommendations also apply to drugs considered to have a “narrow therapeutic index” for which the gap between the therapeutic and toxic dose is small.
Salient Issues Concerning Therapeutic Equivalence and Bioequivalence
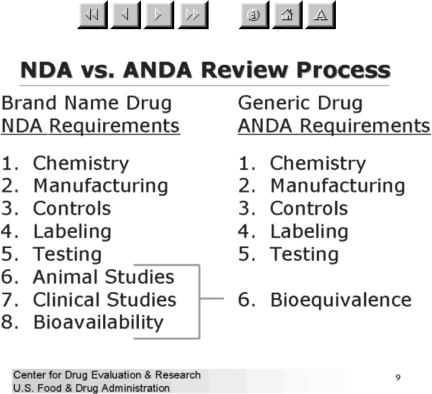
The FDA designates a generic drug as therapeutically equivalent to the reference compound (usually the brand-name drug) if it contains an identical amount of active ingredient in the same dosage form and meets the equivalent standards for strength, quality, purity, and identity (4) (Figure 1) (5). Two pharmacokinetic measures, the area-under-the-curve (AUC) of the drug concentration-time curve and the maximum plasma concentration (Cmax), are used to determine bioequivalence. Equivalence is established when the 90% confidence interval of the ratio of the generic to reference compound for the AUC and Cmax fall within 80–125% range. The in vivo bioequivalence testing is typically performed on 24–36 healthy adults. Although a range of 80–125% in these pharmacokinetic parameters seems quite wide, the important issue is that the 90% confidence interval of the parameters must fall within this range. The FDA reported that it had undertaken reviews in 1987 and again in 1997 of bioequivalence studies and found that the mean difference between the reference and generic compounds was 3.5% for AUC and Cmax in 224 studies carried out between 1964 and 1984. Furthermore, for 127 bioequivalence studies performed on generic drugs approved in 1997, the mean difference between the reference and generic formulations was 3.5% for AUC and 4.3% for Cmax. The FDA contends that it has no conclusive evidence that patients have experienced a difference in adverse effects or seizure control from changes in drug plasma concentration of generic drugs formulated within the bounds it sets for bioequivalence.
FIGURE 1.
NDA versus ANDA review process.
Therapeutic equivalence and bioequivalence are closely related but differ in important respects and that difference may prove to be part of the disagreement between the FDA and many patients or providers regarding the efficacy of generic drugs. The FDA defines bioequivalence as “pharmaceutical equivalents whose rate and extent of absorption are not statistically different when administered to patients or subjects at the same molar dose under similar experimental conditions” (6). Bioequivalence implies the reference and generic drug reach the blood at comparable concentrations over time and depends on equivalence of selected pharmacokinetic parameters. Therapeutic equivalence implies that the reference and generic drugs will provide equal therapeutic effect and depends on the equivalence of selected clinical pharmacodynamic parameters (e.g., efficacy or tolerability). Like bioequivalence, therapeutic equivalence assumes that the reference and generic drug formulations reach the blood in comparable concentrations, but further assumes that the two formulations will exert equal therapeutic or toxic effects. For example, in the case of epilepsy treatment, therapeutic equivalence means a generic and a brand-name antiepileptic drug (AED) formulation will be identical in controlling seizures and avoiding adverse effects. Therapeutic equivalence is not directly tested by the FDA but is presumed based on tests of bioequivalence.
Clinicians Question Whether AED Therapeutic Equivalence Actually Exists
Many health-care providers, patient support organizations, and professional organizations believe that people with epilepsy have an unacceptable incidence of seizures or side effects when switching to generic formulations. What findings form the basis of these beliefs? Wilner reported on survey results from 301 responding neurologists (4.7% response rate): 68% reported seeing breakthrough seizures, and 56% reported increased side effects after a switch from a brand name to a generic AED (7). An electronic survey with 480 responding physicians in Germany, Austria, and Switzerland (21.6% response rate) found that 49% reported problems when switching from a branded to a generic AED (8). Survey data do not provide strong evidence for generic failures, but rather document rough estimates of the extent of physician concern. Case reports document problems related to generic AED substitution in individual patients (9,10). Jain ascertained that 26 of 131 cases of carbamazepine failure reported to the drug maker were associated with seizure increases occurring with a switch to a generic formulation and for which seizure control returned to baseline when the brand formulation was reinstituted (11). A review of generic substitutions for patients with arrhythmias cites similar concerns (12). The FDA has received many reports of generic inequivalence to AED formulations via its voluntary reporting system MedWatch. The FDA acknowledges these reports but notes that the reports are never sufficiently detailed to exclude other possible causes of seizures or adverse effects.
More indirect evidence of generic formulation inequivalence was reported for large numbers of patients who had brand-name products substituted for generic formulations. Andermann et al. reported on switchback rates for people with epilepsy receiving lamotrigine after the Canadian health system urged providers to switch to generic formulations (13). The investigators measured the frequency at which patients returned to brand-name drugs following the widespread introduction of generic formulations of lamotrigine, clobazam, valproate, simvastatin (a statin), and two antidepressants fluoxetine and citalopram. Among 1,354 patients prescribed generic lamotrigine, 12.9% switched back to the brand name, with switchback rates of ∼20% for the other AEDs. In the non-AED group, the switchback rate was only 1.5–2.9%. Significant increases in lamotrigine doses were observed among those who did not switch back to the brand formulation. The primary criticism of the Andermann et al. study is the nonrandomized and unblinded methods used, which could have allowed a substantial contribution of physician and patient bias against generics to potentially influence the outcome. However, it is unclear why such a bias would have more influence in people with epilepsy as compared to those with depression or hyperlipidemia.
Subtle differences in pharmacokinetic parameters between two formulations could produce clinically important differences in adverse effects or seizure control. For instance, Ficker et al. reported that subjects who received an extended release formulation of carbamazepine in an open label trial had significantly lower scores on an inventory of adverse effects as well as an improved quality of life (14). Olling et al. compared pharmacokinetic parameters and side effects among three generic carbamazepine formulations approved in The Netherlands with a brand formulation; they found that the occurrence of side effects, especially dizziness, was associated with differences in absorption rate of the products (15). Mayer et al. compared patients who were receiving a generic extended-release carbamazepine formulations with patients taking a brand formulation in an unblinded trial and found that 9 of 13 subjects experienced adverse effects on the generic formulation, with AUC fluctuations that are acceptable within current FDA guidelines (16). Mayer also reported a single subject who experienced carbamazepine adverse effects with an increase in Cmax of less than 10%. Concentration changes capable of producing adverse clinical symptoms are likely to depend on the plasma concentration range. For example, a patient who experiences a 10% increase in Cmax with a formulation change is more likely to experience adverse effects if the initial concentration is 12 mcg/mL as compared with a patient whose initial concentration is 6 mcg/mL. Detecting differences among adverse effects that are due to formulation changes likely will differ according to the adverse effect—subtle differences between two formulations in the level of fatigue will be more difficult to identify than the presence or absence of diplopia or dizziness.
Potential Factors Contributing to the Lack of AED Therapeutic Equivalence
Health-care providers and patients are concerned because of the impact even a single seizure could have on quality of life. People with well-controlled epilepsy may be most vulnerable, because a single seizure could jeopardize driving privileges or work. A person who has not had a seizure in several years may be more likely to be engaged in potentially dangerous activities (e.g., driving) should a seizure occurred.
If there are truly bioequivalence and therapeutic equivalence among branded and approved generic formulations, why would health-care providers and patients be reporting so many problems? A nocebo effect (i.e., negative symptoms from an inert treatment) involving generic substitutions might be involved. If patients are warned by caregivers or via other medical information sources that generic formulations may be less effective than the brand-name product, patients may experience adverse effects. In addition, patients may be more attentive to adverse effects or more diligent about counting or reporting seizures. In epilepsy clinical trials, placebo groups often experience changes in seizure frequency or adverse effects compared to baseline. For example, in a pooled analysis of topiramate trials, among 265 subjects with refractory epilepsy who received placebo, 15% reported >50% reduction in seizures and 3% withdrew prematurely from the trial as a result of adverse effects (17). Lack of sleep and self-reported stress and anxiety levels were associated with seizure occurrence in a study by Haut et al. (18). It is feasible that a person with epilepsy who believes he or she may be receiving a less effective generic formulation could experience anxiety and stress leading to a seizure. In this situation, the seizure could be due to the generic switch but unrelated to a shift in bioequivalence or therapeutic equivalence.
If generic AED formulations are sometimes not bioequivalent to the brand or other generic formulations, might there be subgroups of people with epilepsy more at risk? Bioequivalence studies are performed with healthy individuals who usually are not taking concomitant medications. Drug–drug interactions, especially interactions that trigger induction of AED metabolism, could be a cause of bio-inequivalence. A recent study compared a lamotrigine immediate-release formulation to an extended-release formulation (19). AUCs were equal for subjects not receiving concomitant medication that induced hepatic enzymes, but AUCs were 20% lower with the extended-release formulation for induced subjects. The Cmax was lower for all subjects who were prescribed the extended-release compared with the immediate-release formulation. This finding was expected, as extended-release formulations are typically designed to reduce peak-to-trough variations in drug concentration and thus decrease adverse effects by lowering the Cmax, while keeping the AUC stable (19). It is possible that people with epilepsy who receive enzyme-inducing medications may be more likely to show bio-inequivalence with generic formulation substitution. This issue is an important concern, because many people with epilepsy are taking multiple AEDs, and the incidence of concomitant medications for comorbid conditions, like depression, is high. Moreover, people with epilepsy at the extremes of age (children or older adults) often have decreases in hepatic enzyme activity and protein binding, potentially making them more susceptible to bio-inequivalence with generic formulation substitution (20).
Looking Forward: Research Needed on Brand and Generic AED Formulations
Studies are needed to determine whether the many complaints about seizures and side effects associated with generic AED formulations are due to bio-inequivalence, therapeutic inequivalence, or other factors (e.g., placebo/nocebo effects, stress, presence of concomitant illness, or progression of underlying neurological illness). The first step is to assess whether problems occurring after generic AED formulations switches are related to bio-inequivalence between the formulations. Such a study might use an enriched population of people with epilepsy who experienced either an increase in seizure frequency or unexpected adverse effects following initiation of a generic AED formulation. Study participants might undergo a single-dose pharmacokinetic trial that involved taking each of the exact same formulations. Any concomitant medications and their dosages would need to be kept stable. Investigators could collect data on adverse effects and seizure frequency, but the primary outcome would be the comparison of pharmacokinetic parameters (i.e., Cmax and AUC). If a substantial number of subjects have Cmax or AUC outside the ranges mandated by the FDA, then the FDA might reconsider its current policy. Further studies to identify patient subgroups or specific factors that may increase the risk of bio-inequivalence with generic formulation substitution would also be valuable. If none of the enriched population of subjects shows Cmax or AUC falling outside the FDA guidelines, then other causes of the many complaints by health-care providers and patients then could be evaluated in a randomized, controlled trial designed to test therapeutic equivalence with additional outcomes of adverse effects and seizure frequency. For example, such a study might determine that some people with epilepsy experience seizures or adverse effects with the small variations in plasma concentration of the magnitude currently allowed by the FDA. The American Epilepsy Society statement on AED formulations generally supports research as suggested here (21).
Until such studies are completed, health-care providers and people with epilepsy would do well to proceed cautiously when switching to generic formulations, with health-care providers communicating to patients the potential risks and benefits of substitution. Extra caution may be needed for patients at highest risk of seizure complications, such as the pregnant patient, patients with recurrent status epilepticus, or patients who have been seizure-free for long periods of time and are driving. These discussions might provide an additional opportunity to advise patients to adhere to AED schedules and timing, avoid seizure triggers (such as alcohol or sleep deprivation), and report to health-care providers any changes in concomitant medication. Physicians who treat epilepsy use the best available scientific evidence in combination with clinical expertise to choose the most appropriate AEDs and dosages for their patient, with the goal of reducing or eliminating seizures while avoiding adverse effects. The American Epilepsy Society's position that formulation substitution should not take place without the physician and patient approval supports this practice (21). The studies outlined in this article provide critical information to resolve much of the controversy surrounding clinical decisions about AED formulation changes.
References
- 1.Savings with generic drugs. Available at http://www.fda.gov/cder/ogd/02-10_BCBS_gjb/sld003.htm Accessed 3/31/2008.
- 2.Liow K, Barkley GL, Pollard JR, Harden CL, Bazil CW, The American Academy of Neurology Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology. 2007;68:1249–1250. doi: 10.1212/01.wnl.0000259400.30539.cc. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein S. Industry fights switch to generics for epilepsy. Wall Street Journal. July 2007;13:A1–A10. [Google Scholar]
- 4.Henney JE. From the food and drug administration. JAMA. 1999;282 1995. [PubMed] [Google Scholar]
- 5.NDA vs. ANDA Review Process. [3/31/2008]; Available at http://www.fda.gov/cder/ogd/02-10_BCBS_gjb/sld009.htm.
- 6.Definition of Bioequivalence. Available at http://www.fda.gov/cder/ogd/02-10_BCBS_gjb/sld028.htm Accessed 3/31/2008.
- 7.Wilner AN. Therapeutic equivalency of generic antiepileptic drugs: results of a survey. Epilepsy Behav. 2004;5:995–998. doi: 10.1016/j.yebeh.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Kramer G, Steinhoff BJ, Feucht M, Pfafflin M, May TW. Experience with generic drugs in epilepsy patients: an electronic survey of members of the German, Austrian and Swiss branches of the ILAE. Epilepsia. 2007;48:609–611. doi: 10.1111/j.1528-1167.2007.01084_1.x. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt RT, Leppik IE, Blesi K, Scott S, Gapany SR, Cloyd JC. Lower phenytoin serum levels in persons switched from brand to generic phenytoin. Neurology. 2004;63:1494–1496. doi: 10.1212/01.wnl.0000142091.47698.a2. [DOI] [PubMed] [Google Scholar]
- 10.Gilman JT, Alvarez LA, Duchowny M. Carbamazepine toxicity resulting from generic substitution. Neurology. 1993;43:2696–2697. doi: 10.1212/wnl.43.12.2696. [DOI] [PubMed] [Google Scholar]
- 11.Jain KK. Investigation and management of loss of efficacy of an antiepileptic medication using carbamazepine as an example. J R Soc Med. 1993;86:133–136. doi: 10.1177/014107689308600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Issues in Bioequivalence and Generic Substitution for Antiarrhythmic Drugs. Available at http://www.americanheart.org/presenter.jhtml?identifier=3015266 Accessed 3/31/2008.
- 13.Andermann F, Duh MS, Gosselin A, Paradis PE. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia. 2007;48:464–469. doi: 10.1111/j.1528-1167.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 14.Ficker DM, Privitera M, Krauss G, Kanner A, Moore JL, Glauser T. Improved tolerability and efficacy in epilepsy patients with extended-release carbamazepine. Neurology. 2005;65:593–595. doi: 10.1212/01.wnl.0000172932.95985.51. [DOI] [PubMed] [Google Scholar]
- 15.Olling M, Mensinga TT, Barends DM, Groen C, Lake OA, Meulenbelt J. Bioavailability of carbamazepine from four different products and the occurrence of side effects. Biopharm Drug Dispos. 1999;20:19–28. doi: 10.1002/(sici)1099-081x(199901)20:1<19::aid-bdd152>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Mayer T, May TW, Altenmuller DM, Sandmann M, Wolf P. Clinical problems with generic antiepileptic drugs: comparison of sustained-release formulations of carbamazepine. Clin Drig Invest. 1999;18:17–26. [Google Scholar]
- 17.Peeters K, Adriaenssen I, Wapenaar R, Neto W, Pledger G. A pooled analysis of adjunctive topiramate in refractory partial epilepsy. Acta Neurol Scand. 2003;108:9–15. doi: 10.1034/j.1600-0404.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 18.Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69:1905–1910. doi: 10.1212/01.wnl.0000278112.48285.84. [DOI] [PubMed] [Google Scholar]
- 19.Tompson DJ, Ali I, Oliver-Willwong R. Steady-state pharmacokinetics of lamotrigine when converting from a twice-daily immediate-release to a once-daily extended-release formulation in subjects with epilepsy (the COMPASS study) Epilepsia. 2008;49:410–417. doi: 10.1111/j.1528-1167.2007.01274.x. [DOI] [PubMed] [Google Scholar]
- 20.Leppik IE. Epilepsy in the elderly. Epilepsia. 2006;47(suppl 1):65–70. doi: 10.1111/j.1528-1167.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 21.Position statement on the substitution of different formulations of antiepileptic drugs for the treatment of epilepsy. [March 31]; Available at http://www.aesnet.org.