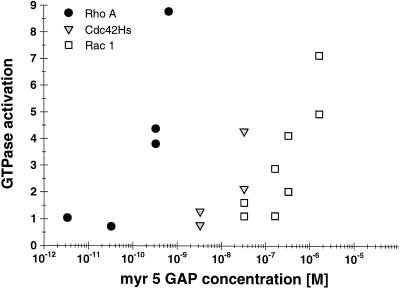
Figure 1.
Differential specificity of the myr 5 GAP domain for RhoA, Cdc42Hs, and Rac1. The myr 5 GAP domain was purified as a GST fusion protein from E. coli and subsequently cleaved from GST by thrombin. Recombinant GTPases were preloaded with [γ-32P]GTP and incubated in the absence or presence of different amounts of myr 5 GAP domain. After 0, 5, and 10 min, aliquots of the reactions were removed and the remaining radioactive GTP bound to the GTPases was determined in a filter binding assay. The decrease in radioactivity was used for the calculation of rate constants for the GTPase activity. GTPase activation by increasing amounts of myr 5 GAP domain was determined for RhoA (•), Cdc42Hs (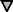 ), and Rac1 (□).
), and Rac1 (□).