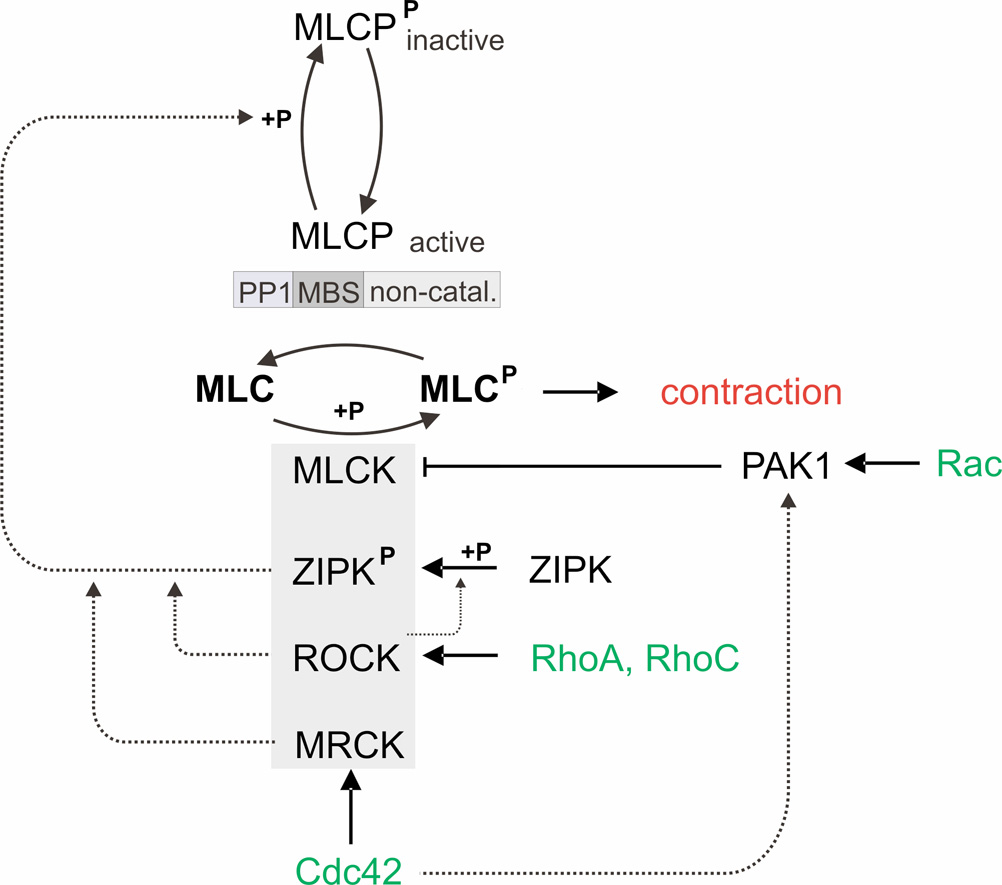
Fig. 3.
Regulation of contractile forces. Contractile forces are controlled by myosin light chain (MLC) phosphorylation, which in turn depends on the balanced activities of the myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP). Force up-regulation: MLCP can be inhibited by phosphorylation either through Rho-associated kinase (ROCK), through zipper-interacting protein kinase (ZIPK), or through myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) (dotted lines). ROCK has been shown to indirectly inhibit MLCP via ZIRP phosphorylation. ROCK is activated by the small GTPases RhoA and RhoC, and MRCK is activated by the small GTPase Cdc42. ZIPK, ROCK and MRCK have also been shown to directly phosphorylate MLC. Force down-regulation: p21-activated protein kinase 1 (PAK1) inhibits MLCK and is activated by the small GTPases Rac and Cdc42.