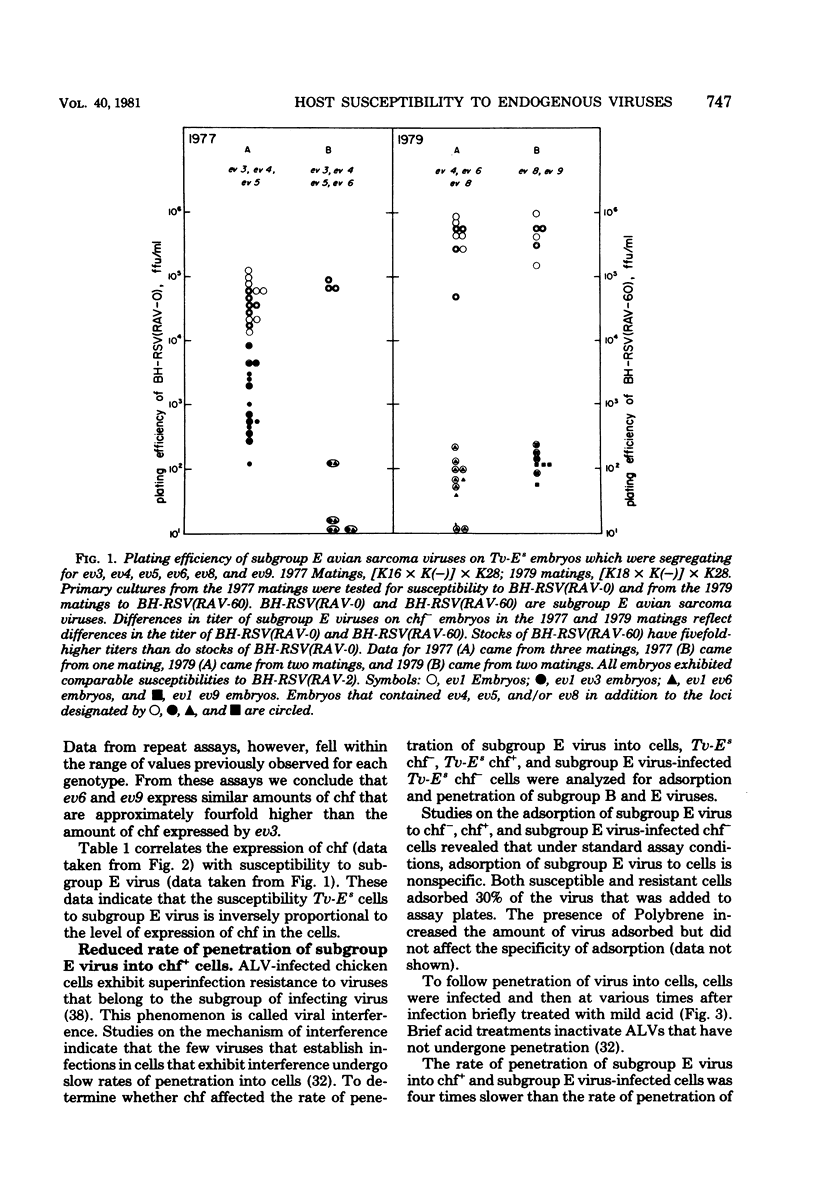
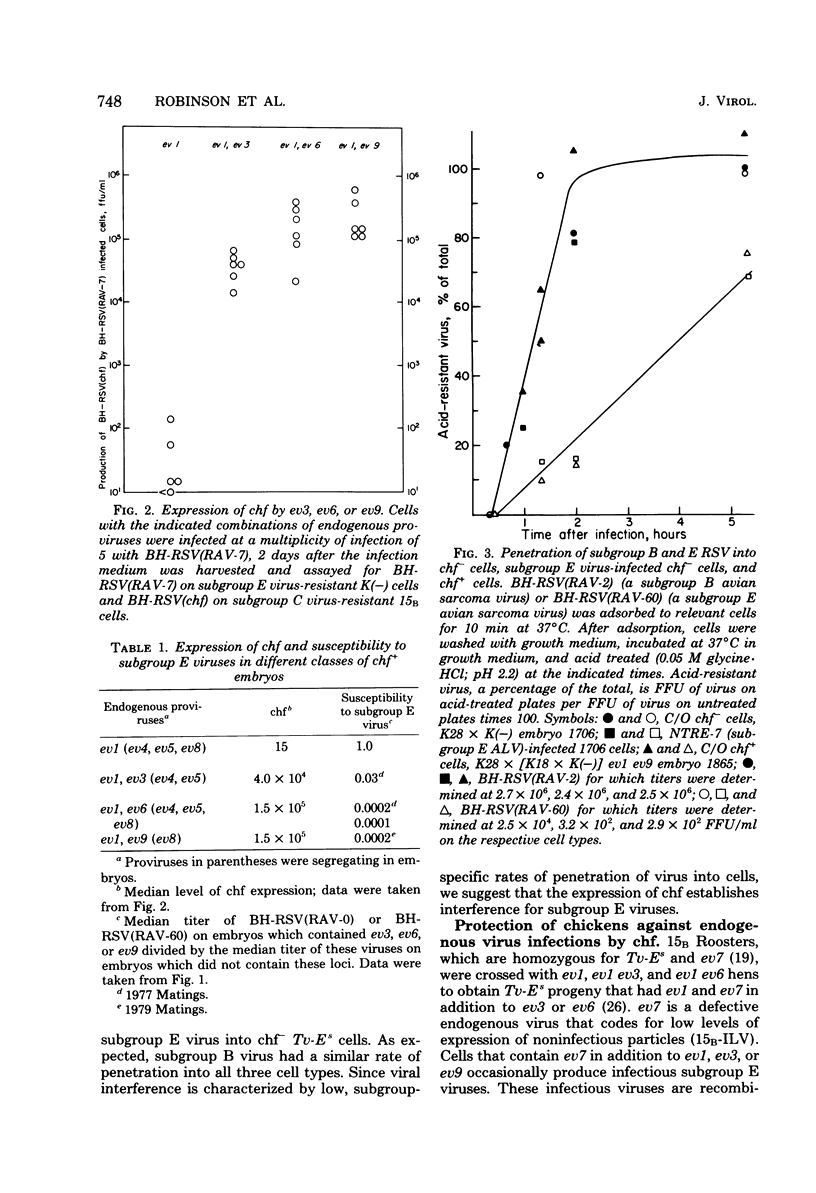
Abstract
Three defective endogenous avian leukosis viruses, ev3, ev6 and ev9, interfered with subgroup E virus infections, ev3, ev6, and ev9 expressed high levels of subgroup E envelope glycoproteins. These glycoproteins reduced the activity of cellular receptors for subgroup E viruses. ev3 and ev6 protected chickens and cultured cells from subgroup E virus infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Toyoshima K. Genetic control of chick helper factor in cells which lack natural group-specific antigen of avian leukosis. Virology. 1976 Sep;73(2):521–527. doi: 10.1016/0042-6822(76)90413-x. [DOI] [PubMed] [Google Scholar]
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L., Crittenden L. B., Buss E. G., Wyban J., Hayward W. S. Ten genetic loci in the chicken that contain structural genes for endogenous avian leukosis viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1105–1109. doi: 10.1101/sqb.1980.044.01.119. [DOI] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L. Gs, an allele of chickens for endogenous avian leukosis viral antigens, segregates with ev 3, a genetic locus that contains structural genes for virus. J Virol. 1979 Aug;31(2):420–425. doi: 10.1128/jvi.31.2.420-425.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B., Motta J. V. The role of the tvb locus in genetic resistance to RSV(RAV-O). Virology. 1975 Oct;67(2):327–334. doi: 10.1016/0042-6822(75)90434-1. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Smith E. J., Gulvas F. A., Robinson H. L. Endogenous virus expression in chicken lines maintained at the Regional Poultry Research Laboratory. Virology. 1979 Jun;95(2):434–444. doi: 10.1016/0042-6822(79)90498-7. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Wendel E. J., Motta J. V. Interaction of genes controlling resistance to RSV(RAV-O). Virology. 1973 Apr;52(2):373–384. doi: 10.1016/0042-6822(73)90332-2. [DOI] [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S., Pal B. K., Estes J. D., O'Brien S. J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc Natl Acad Sci U S A. 1980 Jan;77(1):531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Friis R. R. Immunogenicity of the envelope glycoprotein of avian sarcoma virus. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1962–1966. doi: 10.1073/pnas.75.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Braverman S. B., Astrin S. M. Transcriptional products and DNA structure of endogenous avian proviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1111–1121. doi: 10.1101/sqb.1980.044.01.120. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Cooper G. M. Integration, expression, and infectivity of exogenously acquired proviruses of Rous-associated virus-O. J Virol. 1980 Dec;36(3):684–691. doi: 10.1128/jvi.36.3.684-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Integration of Moloney leukaemia virus into the germ line of mice: correlation between site of integration and virus activation. Nature. 1980 Oct 2;287(5781):456–458. doi: 10.1038/287456a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta J. V., Crittenden L. B., Purchase H. G., Stone H. A., Witter R. L. Low oncogenic potential of avian endogenous RNA tumor virus infection or expression. J Natl Cancer Inst. 1975 Sep;55(3):685–689. doi: 10.1093/jnci/55.3.685. [DOI] [PubMed] [Google Scholar]
- Obata Y., Ikeda H., Stockert E., Boyse E. A. Relation of GIX antigen of thymocytes to envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):188–197. doi: 10.1084/jem.141.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani P. K., Payne L. N. Further evidence for two loci which control susceptibility of fowl to RSV(RAV-O). J Gen Virol. 1973 May;19(2):235–244. doi: 10.1099/0022-1317-19-2-235. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Pani P. K., Weiss R. A. A dominant epistatic gene which inhibits cellular susceptibility to RSV(RAV-O). J Gen Virol. 1971 Dec;13(3):455–462. doi: 10.1099/0022-1317-13-3-455. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Salazar F. H. V-15B, an allele of chickens for the production of a noninfectious avian leukosis virus. Virology. 1979 Nov;99(1):10–20. doi: 10.1016/0042-6822(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Eisenman R., Senior A., Ripley S. Low freqeuncy production of recombinant subgroup E avian leukosis viruses by uninfected V-15B chicken cells. Virology. 1979 Nov;99(1):21–30. doi: 10.1016/0042-6822(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978;83:1–36. doi: 10.1007/978-3-642-67087-9_1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Intracellular restriction on the growth of induced subgroup E avian type C viruses in chicken cells. J Virol. 1976 Jun;18(3):856–866. doi: 10.1128/jvi.18.3.856-866.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Lamoreux W. F. Expression of endogenous ALV antigens and susceptibility to subgroup E ALV in three strains of chickens (endogenous avian C-type virus). Virology. 1976 Jan;69(1):50–62. doi: 10.1016/0042-6822(76)90193-8. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Crittenden L. B., Whitson A. K. Radioimmunoassay for the envelope glycoprotein of subgroup E avian leukosis-sarcoma viruses. Virology. 1978 Feb;84(2):331–340. doi: 10.1016/0042-6822(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976 Nov;75(1):130–144. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. FV-4: a new gene affecting the splenomegaly induction by Friend leukemia virus. Jpn J Exp Med. 1975 Dec;45(6):473–478. [PubMed] [Google Scholar]
- Tereba A., Astrin S. M. Chromosomal localization of ev-1, a frequently occurring endogenous retrovirus locus in white Leghorn chickens, by in situ hybridization. J Virol. 1980 Sep;35(3):888–894. doi: 10.1128/jvi.35.3.888-894.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Conklin K. F., Coffin J. M. Mutant and recombinant avian retroviruses with extended host range. Proc Natl Acad Sci U S A. 1980 Jan;77(1):536–540. doi: 10.1073/pnas.77.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- Wong P. K., McCarter J. A. Studies of two temperature-sensitive mutants of Moloney murine leukemia virus. Virology. 1974 Apr;58(2):396–408. doi: 10.1016/0042-6822(74)90075-0. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Naito Y., Moriwaki K. Unstable resistance of G mouse fibroblasts to ecotropic murine leukemia virus infection. J Virol. 1979 Mar;29(3):1078–1086. doi: 10.1128/jvi.29.3.1078-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


