Figure 1.
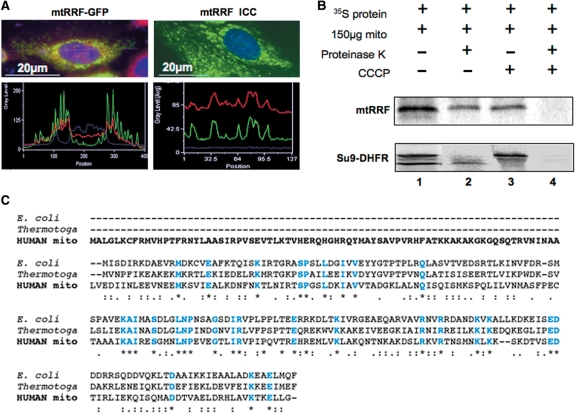
mtRRF is a mitochondrial protein with an extended N-terminal pre-sequence. (A) Human mtRRF is targeted to mitochondria. Left panels show HeLa cells transiently transfected (24 h) with a mtRRF–GFP fusion construct. Cells were stained to visualize nuclei (DAPI blue) and mitochondria (Mitotracker red). Fluorescence images and linescans confirmed mitochondrial localization of mtRRF–GFP by superimposition of green and red signals (lower left). The image reflects three-independent transfections. Endogenous mtRRF in HeLa cells was visualized by immunocytochemistry (upper right) using affinity purified anti-mtRRF and FITC secondary. DAPI-stained nuclei (blue) and Mitotracker the mitochondria (red). Mitochondrial localization of mtRRF was confirmed by superimposition of linescan green and red fluorescence (lower right). The image reflects three-independent transfections. (B) Human mtRRF is imported into mitochondria. FL 35S-radiolabelled mtRRF was in vitro synthesized and incubated with rat liver mitochondria (lane 1). Under import conditions a single product is visible (lane 2) that is protected from proteinase K (lane 3), but degraded by treatment with proteinase K and FCCP uncoupler (lane 4). Control import reactions contained DHFR with a mitochondrial pre-sequence (Su9) showing the FL pre-protein and the matured form under import conditions. (C) Sequence alignment (CLUSTALW) indicates an extensive N-terminal pre-sequence when compared with E. coli or Thermotoga maritima. Identity to these RRFs is indicated in blue and by *, high levels of similarity by a colon ‘:’ and lower levels by a fullstop ‘.’.