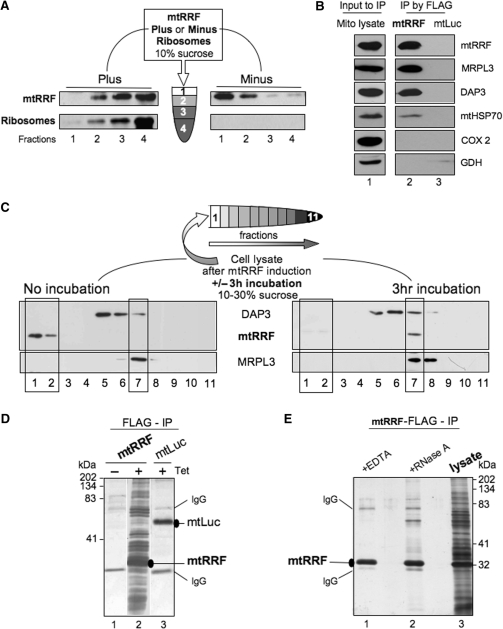
Figure 2.
Human mtRRF associates with bacterial ribosomes in vitro and mitoribosomes in vivo. (A) Human mtRRF binds E. coli ribosomes. Recombinant mtRRF (mtRRFΔ69) was incubated with or without 70S ribosomes prior to sedimentation through 10% (w:v) sucrose. Fractions were subjected to western analysis. (B) mtRRF associates with mitoribosomes. FLAG-mtRRF and mtLuc HEK293T cells were induced, mitochondria isolated. Lysate was prepared (lane 1) from which proteins were immunoprecipitated via the FLAG epitope (lanes 2–3). Western blot analysis used antibodies to mtRRF, large 39S and small 28S mitoribosomal subunits (MRPL3, DAP3), mitochondrial chaperone (mtHSP70), complex IV (COX 2) and a matrix protein (glutamate dehydrogenase -GDH). (C) Pre-incubation of mitochondria allows detection of mtRRF binding to mitoribosomes. Lysate was prepared from HEK293T cells after induction of FLAG-mtRRF and was either separated immediately or post 3 h incubation on a 10–30% (v:v) isokinetic sucrose gradient. Fractions were analysed by western blot with antibodies to mtRRF, MRPL3 (39S mitoribosomal subunit) or DAP3 (28S mitoribosomal subunit). A similar time-dependent mitoribosomal association in HeLa cell lysate was noted for the endogenous mtRRF (data not shown). (D) HEK293T cells were induced (±Tet) for FLAG-mtRRF (lanes 1–2) or control mtluc (lane 3) expression and mitochondria isolated. Co-immunoprecipitating proteins were separated through a 12% SDS–PAG and visualized by silver stain. mtRRF, mtluc and anti-FLAG IgG are indicated. (E) Preparations of the mtRRF IP were also treated with EDTA (lane 1) or RNase A (lane 2). Mitochondrial lysate is shown in lane 3.