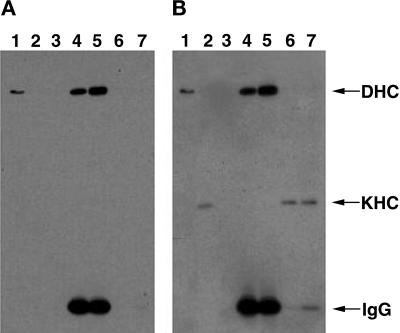
Figure 4.
Immunoprecipitation of cDHC does not coprecipitate KHC. Drosophila cytosol fractions were separated by gel electrophoresis and transferred to a membrane. The membrane was stained first for cDHC (A) and subsequently for KHC (B). Thus, the cDHC bands are present in both panels. The experiment shown here was conducted with ovary cytosol from DHC-3HA–expressing flies immunoprecipitated with anti-HA mAb–agarose bead complexes. However, comparable results were obtained with DHC-3HA head extracts and either ovary or head wild-type extracts with the use of DHC mAbs for the immunoprecipitation. (Lanes 1) Proteins that pelleted from cytosol with taxol-stabilized microtubules in the absence of ATP. cDHC but not KHC was efficiently bound to microtubules under these conditions. (Lanes 2) KHC but not DHC was readily detected in cytosol. (Lanes 3) The pellet from cytosol that was incubated with unconjugated agarose beads. (Lanes 4 and 5) The pellets from cytosol that was incubated with beads conjugated with antibodies to cDHC via the 3HA tag; incubation was at either 4°C (lanes 4) or 25°C (lanes 5). (Lanes 6 and 7) The supernatants of the pellets shown in lanes 4 and 5, respectively. KHC remained in the immunoprecipitation supernatants, and cDHC was detected in the pellets. IgG, immunoglobulin G.