Figure 1.
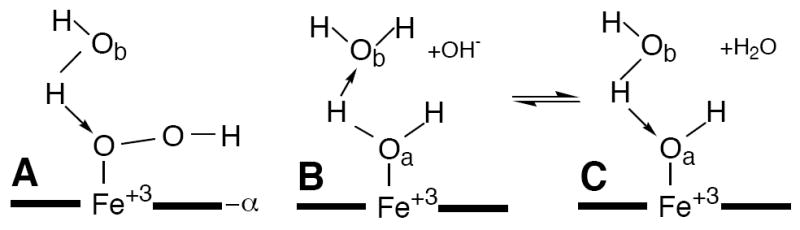
Geometry of a ferric porphyrin ligated: (A) hydroperoxide; (B) neutral water molecule a; and (C) hydroxide. The latter two species are at equilibrium at any solution pH value; this equilibrium shifts to the right with increasing pH. Non-ligated water molecule (b) in the distal pocket provides the major interaction for the axially ligated water molecule 14 serving as a donor to molecule b (B). Both the hydroperoxide (A), and hydroxide (C) serve as acceptors to water b.
