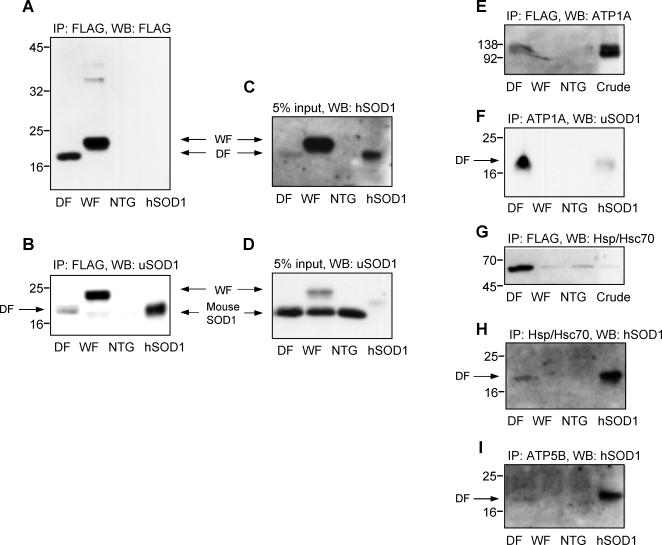
Figure 2. Immunoprecipitation and western blot analyses of mouse spinal cords.
The FLAG-tagged SOD1 and the ligand proteins were immunoprecipitated using the FLAG affinity resin and detected by the anti FLAG antibody (A) and anti-SOD1 polyclonal antibody (uSOD1) that recognizes both human and mouse SOD1 (B). The DF mutant SOD1 shows a distinct single band (A and B). In contrast, the WF shows high molecular weight bands (A) and a low molecular weight band which is possibly the mouse intrinsic SOD1 (B), indicating that the WF forms the heterodimer with the mouse SOD1. Crude spinal cord extracts (5% input) were analyzed using antibodies for human specific SOD1 (hSOD1) (C) and for uSOD1 (D). Compared to the WF, the amount of the DF SOD1 protein is considerably lower (C). The WF itself is lower still compared to the mouse intrinsic SOD1 (D). The immunoprecipitation products with the FLAG were analyzed by antibodies for ATP1A (E) and Hsp/Hsc70 (G). In this analysis, only the DF preparations show the presence of ATP1A (E). In the Hsp/Hsc70 analysis in (G), there are non-specific precipitations also in WF and NTG. In contrast, the immunoprecipitated samples for ATP1A (F), Hsp/Hsc70 (H) and ATP5B (I) were analyzed using antibodies for hSOD1 (F) or uSOD1 (H and I). Correspondingly, the human form of SOD1 is only seen to be co-immunoprecipitated in DF preparation (F, H and I). Lanes from the left are: DF, WF, NTG, and a purified human SOD1 (A, B, C, D, F, H, and I); and DF, WF, NTG, and a crude soluble fraction of NTG mice (E and G).