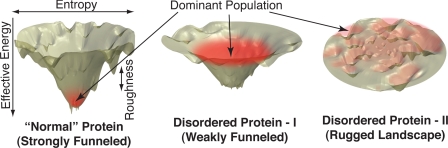
Fig. 1.
The interplay between protein's folding and functional landscapes. (Left) Energy landscapes of globular proteins are thought to be funneled, such that the native state is both a thermodynamic global minimum and is also kinetically accessible (11). It is a common practice to partition the total free energy into protein chain's structural entropy (horizontal axes) and the remaining part, which is often called “effective energy,” or simply energy (vertical axes). Note that the latter also includes entropic contributions, mainly from the solvent. (Center) Energy landscapes of many disordered proteins are likely organized around a special state, characterized by a weakly funneled landscape (6). However, the driving force for folding is too weak, allowing the protein to remain disordered. Interactions with specific targets create additional favorable contacts, deepening the funnel and driving subsequent folding (6). Transient population of catalytically competent states, near the funnel's bottom, may allow for efficient catalysis (9). (Right) A random energy landscape is shown, where search for a specific functionally competent conformation is extremely inefficient.